Stable Nickel-Based Metal–Organic Framework Containing Thiophene/Diimidazole Units for Effective Near-Infrared Photothermal Conversion
Abstract
:1. Introduction
2. Results
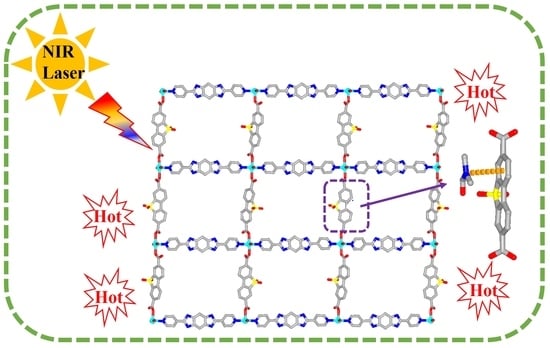
2.1. The Structure of Complex 1
2.2. The PXRD and TGA Exploration of Complex 1
2.3. The IR and UV/Vis-NIR Spectra of Complex 1
2.4. The Near-Infrared Photothermal Conversion Studies
3. Experiment
3.1. Materials and Methods
3.2. Synthesis of Complex 1
3.3. X-ray Crystallography
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lyu, Y.; Fang, Y.; Miao, Q.Q.; Zhen, X.; Ding, D.; Pu, K.Y. Intraparticle molecular orbital engineering of semiconducting polymer nanoparticles as amplified theranostics for in vivo photoacoustic imaging and photothermal therapy. ACS Nano 2016, 10, 4472–4481. [Google Scholar] [CrossRef]
- Pu, K.; Shuhendler, A.J.; Jokerst, J.V.; Mei, J.G.; Gambhir, S.S.; Bao, Z.N.; Rao, J.H. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nano 2014, 9, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Liang, C.; Sun, X.Q.; Chen, J.W.; Yang, Z.J.; Zhao, H.; Feng, L.Z.; Liu, Z. H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proc. Natl. Acad. Sci. USA 2017, 114, 5343–5348. [Google Scholar] [CrossRef] [Green Version]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.L.; Kim, C.; Rubinstein, J.L.; Chan, W.C.W.; Cao, W.G.; Wang, L.V.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.R.; Adams, R.L.; Higgins, A.K.; Bartels, K.E.; Nordquist, R.E. Photothermal effects on murine mammary tumors using indocyanine green and an 808-nm diode laser: An in vivo efficacy study. Cancer Lett. 1996, 98, 169–173. [Google Scholar] [CrossRef]
- Yang, J.; Choi, J.; Bang, D.; Kim, E.; Lim, E.K.; Park, H.; Suh, J.S.; Lee, K.; Yoo, K.H.; Kim, E.K.; et al. Convertible organic nanoparticles for near-Infrared photothermal ablation of cancer cells. Angew. Chem. Int. Ed. 2011, 50, 441–444. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Sun, D.D.; Zhang, B.; Sun, Q.Q.; Zhang, Y.; Liu, S.R.; Wang, Y.M.; Liu, C.T.; Chen, J.Z.; Chen, J.B.; et al. Intrinsic carbon nanotube liquid crystalline elastomer photoactuators for high-definition biomechanics. Mater. Horiz. 2022, 9, 1045–1056. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.G.; Du, W.N.; Liu, X.F.; Zhang, X.T.; Dong, H.L.; Hu, W.P. Cocrystals strategy towards materials for near-infrared photothermal conversion and imaging. Angew. Chem. Int. Ed. 2018, 57, 3963–3967. [Google Scholar] [CrossRef]
- Song, Q.; Jiao, Y.; Wang, Z.Q.; Zhang, X. Tuning the energy gap by supramolecular approaches: Towards near-infrared organic assemblies and materials. Small 2016, 12, 24–31. [Google Scholar] [CrossRef]
- Qian, G.; Wang, Z.Y. Near-infrared organic compounds and emerging applications. Chem. Asian J. 2010, 5, 1006–1029. [Google Scholar] [CrossRef]
- Li, T.T.; Dang, L.L.; Zhao, C.C.; Lv, Z.Y.; Yang, X.G.; Zhao, Y.; Zhang, S.H. A self-sensitized Co (II)-MOF for efficient visible-light-driven hydrogen evolution without additional cocatalysts. J. Solid State Chem. 2021, 304, 122609–122614. [Google Scholar] [CrossRef]
- Qin, J.H.; Xu, P.; Huang, Y.D.; Xiao, L.Y.; Lu, W.W.; Yang, X.G.; Ma, L.F.; Zang, S.S. High loading of Mn(II)-metalated porphyrin in MOF for photocatalytic CO2 reduction in gas−solid condition. Chem. Commun. 2021, 57, 8468–8471. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef]
- Chang, X.H.; Qin, W.J.; Zhang, X.Y.; Jin, X.; Yang, X.G.; Dou, C.X.; Ma, L.F. Angle-Dependent polarized emission and photoelectron performance of dye-encapsulated metal-organic framework. Inorg. Chem. 2021, 60, 10109–10113. [Google Scholar] [CrossRef]
- Yang, X.G.; Zhai, Z.M.; Lu, X.M.; Qin, J.H.; Li, F.F.; Ma, L.F. Hexanuclear Zn(II)-Induced dense π-Stacking in a metal–organic framework featuring Long-Lasting room temperature phosphorescence. Inorg. Chem. 2020, 59, 10395–10399. [Google Scholar] [CrossRef]
- Chang, X.H.; Ling, X.L.; Lu, X.M.; Yang, X.G.; Li, F.F.; Guo, Y.M. Near-infrared phosphorescence emission of three-fold interpenetrated MOF based on 1,4-bis(imidazole-1-ylmethyl)benzene: Syntheses, structure and photoelectron performance. J. Solid State Chem. 2020, 292, 121694. [Google Scholar] [CrossRef]
- Zeng, L.; Guo, X.Y.; He, C.; Duan, C.Y. Metal-organic frameworks: Versatile materials for heterogeneous photocatalysis. ACS Catal. 2016, 6, 7935–7947. [Google Scholar] [CrossRef]
- Luo, F.; Yang, C.S.; Dang, L.L.; Krishna, R.; Zhou, W.; Wu, H.; Dong, X.L.; Han, Y.; Hu, T.L.; Keeffe, M.Z.; et al. UTSA-74: A MOF-74 isomer with two accessible binding sites per metal center for highly selective gas separation. J. Am. Chem. Soc. 2016, 138, 5678–5684. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Huang, R.K.; He, C.T.; Gong, L.; Hu, Q.; Wang, L.S.; Xu, Y.T.; Tian, X.Y.; Liu, S.Y.; et al. Electrochemical exfoliation of pillared-layer metal−organic framework to boost the oxygen evolution reaction. Angew. Chem. 2018, 130, 4722–4726. [Google Scholar] [CrossRef]
- Böhme, U.; Barth, B.; Paula, C.; Kuhnt, A.; Schwieger, W.; Mundstock, A.; Caro, J.; Hartmann, M. Ethene/ethane and propene/propane separation via the olefifin and paraffifin selective metal–organic framework adsorbents CPO-27 and ZIF- 8. Langmuir 2013, 29, 8592–8600. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, Z.W.; Liu, F.Q.; Yu, Y.; Su, X.M.; Wang, L.; Xu, Z.Z.; Yang, Y.L.; Wu, G.R.; Feng, X.F.; et al. Ultralow content iron-decorated Ni-MOF-74 fabricated by a metal-organic framework surface reaction for efficient electrocatalytic water oxidation. Inorg. Chem. 2019, 58, 11500–11507. [Google Scholar] [CrossRef]
- Li, H.L.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, Y.J.; Wang, N.; Zheng, P.; Fu, H.R.; Han, M.L.; Ma, L.F.; Wang, L.Y. Tetraphenylethylene-Decorated Metal−Organic Frameworks as Energy-Transfer Platform for the Detection of Nitro-Antibiotics and White-Light Emission. Inorg. Chem. 2019, 58, 12700–12706. [Google Scholar] [CrossRef]
- Dang, L.L.; Li, T.T.; Cui, Z.; Sui, D.; Ma, L.F.; Jin, G.X. Selective construction and stability studies of a molecular trefoil knot and Solomon link. Dalton Trans. 2021, 50, 16984–16989. [Google Scholar] [CrossRef]
- Yang, X.G.; Liu, X.Y.; Zhai, Z.M.; Qin, J.H.; Chang, X.H.; Han, M.L.; Li, F.F.; Ma, L.F. π-Type halogen bonding enhanced long-last room temperature phosphorescence of Zn(II) coordination polymers for photoelectron response applications. Inorg. Chem. Front. 2020, 7, 2224–2230. [Google Scholar] [CrossRef]
- Dang, L.L.; Li, T.T.; Zhao, C.C.; Zhang, T.T.; Ye, X.Y.; Sun, X.T.; Wang, H.R.; Ma, L.F. Supramolecular Rh6 catalytic system promoting directed [4+4] cycloaddition reaction of anthracene under UV irradiation. J. Solid State Chem. 2022, 306, 122785–122792. [Google Scholar] [CrossRef]
- Qin, J.H.; Huang, Y.D.; Zhao, Y.; Yang, X.G.; Li, F.F.; Wang, C.; Ma, L.F. Highly dense packing of chromophoric linkers achievable in a Pyrene-Based Metal–Organic framework for photoelectric response. Inorg. Chem. 2019, 58, 15013–15016. [Google Scholar] [CrossRef]
- Wang, H.R.; Yang, X.G.; Qin, J.H.; Ma, L.F. Long-lived room temperature phosphorescence of organic–inorganic hybrid systems. Inorg. Chem. Front. 2021, 8, 1942–1950. [Google Scholar] [CrossRef]
- Wang, H.; Meng, W.; Wu, J.; Ding, J.; Hou, H.; Fan, Y. Crystalline central-metal transformation in metal-organic frameworks. Coord. Chem. Rev. 2016, 307, 130–146. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, J.W.; Jiang, X.; Chen, J.; Chen, T.W.; Chen, K.J. Band gap modulation enabled by TCNQ loading in a Ru-based metal–organic framework for enhanced near-infrared absorption and photothermal conversion. Cryst. Growth Des. 2021, 21, 729–734. [Google Scholar] [CrossRef]
- Yan, T.; Li, Y.Y.; Su, J.; Wang, H.Y.; Zuo, J.L. Charge transfer metal-organic framework containing redox-active TTF/NDI units for highly efficient near-infrared photothermal conversion. Chem. Eur. J. 2021, 27, 11050–11055. [Google Scholar] [CrossRef]
- Lü, B.Z.; Chen, Y.F.; Li, P.Y.; Wang, B.; Müllen, K.; Yin, M.Z. Stable radical anions generated from a porous perylenediimide metal-organic framework for boosting near-infrared photothermal conversion. Nat. Commun. 2019, 10, 767. [Google Scholar] [CrossRef]
- Liu, Y.G.; Liu, G.L.; Peng, T.; Gu, C.; Gu, C.; Li, J.J.; Liu, X.Q.; Sun, L.B. Near-infrared light triggered release of ethane from a photothermal metal-organic framework. Chem. Eng. J. 2021, 420, 130490. [Google Scholar] [CrossRef]
- Dang, L.L.; Li, T.T.; Zhang, T.T.; Zhao, Y.; Chen, T.; Gao, X.; Ma, L.F.; Jin, G.X. Highly selective synthesis and near-infrared photothermal conversion of metalla-Borromean ring and [2]catenane assemblies. Chem. Sci. 2022, 13, 5130–5140. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, S.H.; Ma, L.F.; Geng, J.L.; Wang, L.Y. Syntheses, crystal structures, and magnetic studies of two cobalt(II) coordination polymers based on concurrent ligand extension. Inorg. Chem. Commun. 2015, 62, 42–46. [Google Scholar] [CrossRef]
- Yan, T.; Li, Y.Y.; Gu, Q.Y.; Li, J.; Su, J.; Wang, H.Y.; Zuo, J.L. A Tetrathiafulvalene/Naphthalene Diimide-Containing Metal–Organic Framework with fsc Topology for Highly Efficient Near-Infrared Photothermal Conversion. Inorg. Chem. 2022, 61, 3078–3085. [Google Scholar] [CrossRef]
- Wu, X.; Fu, H.; Han, M.; Zhou, L.; Ma, L. Tetraphenylethylene Immobilized Metal–Organic Frameworks: Highly Sensitive Fluorescent Sensor for the Detection of Cr2O72– and Nitroaromatic Explosives. Cryst. Growth Des. 2017, 17, 6041–6048. [Google Scholar] [CrossRef]
- Chang, X.H.; Qin, J.H.; Han, M.L.; Ma, L.F.; Wang, L.Y. Exploring the structural diversities and magnetic properties of copper(II) and manganese(II) complexes based on 5-methoxyisophthalate and flexible bis(imidazole) ligands. CrystEngComm 2014, 16, 870–882. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Peng, F.; Meng, F.; Zha, J.J.; Ma, L.; Du, Y.H.; Peng, N.; Ma, L.F.; Zhang, Q.H.; et al. Intercalation-activated layered MoO3 nanobelts as biodegradable nanozymes for tumor-specific photo-enhanced catalytic therapy. Angew. Chem. Int. Ed. 2022, 61, e202115939. [Google Scholar]
- Yang, X.G.; Zhai, Z.M.; Lu, X.M.; Ma, L.F.; Yan, D.P. Fast crystallization-deposition of orderly molecule level heterojunction thin films showing tunable up-conversion and ultrahigh photoelectric response. ACS Cent. Sci. 2020, 6, 1169–1178. [Google Scholar] [CrossRef]
- Yang, X.G.; Qin, J.H.; Huang, Y.D.; Zhai, Z.M.; Ma, L.F.; Yan, D.P. Highly enhanced UV-vis-NIR light harvesting and photoelectric conversion of pyrene MOF by encapsulation of D-π-A cyanine dye. J. Mater. Chem. C 2020, 8, 17169–17175. [Google Scholar] [CrossRef]
- Dang, L.L.; Chen, T.; Zhang, T.T.; Li, T.T.; Song, J.L.; Zhang, K.J.; Ma, L.F. Size-Induced Highly Selective Synthesis of Organometallic Rectangular Macrocycles and Heterometallic Cage Based on Half-Sandwich Rhodium Building Block. Molecules 2022, 27, 3756. [Google Scholar] [CrossRef]
- Xue, X.; Wang, H.; Han, Y.; Hou, H. Photoswitchable nonlinear optical properties of metal complexes. Dalton Trans. 2018, 47, 13–22. [Google Scholar] [CrossRef]
- Qin, J.H.; Zhang, H.; Sun, P.; Huang, Y.D.; Shen, Q.; Yang, X.G.; Ma, L.F. Ionic liquid induced highly dense assembly of porphyrin in MOF nanosheets for photodynamic therapy. Dalton Trans. 2020, 49, 17772–17778. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Z.; Shen, Y.R.; Liu, D.; Lin, Y.J.; Jin, G.X. Synthesis and Near-Infrared photothermal conversion of discrete supramolecular topologies featuring Half-Sandwich [Cp*Rh] units. J. Am. Chem. Soc. 2021, 143, 17833–17842. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, D.; Lin, Y.J.; Jin, G.X. A Hierarchical Assembly Strategy for Near-Infrared Photothermal Conversion: Unconventional Heterogeneous Metalla[2]catenanes. Chem. Sci. 2020, 11, 11509–11513. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.G.; Lu, X.M.; Yang, C.D.; Fan, N.N.; Yang, Z.T.; Wang, L.Y.; Ma, L.F. {Zn6} Cluster Based Metal–Organic Framework with Enhanced Room-Temperature Phosphorescence and Optoelectronic Performances. Inorg. Chem. 2019, 58, 6215–6221. [Google Scholar] [CrossRef]
- Zhang, T.T.; Chen, T.; Dang, L.L.; Li, T.T.; Sun, K.X.; Gao, Y.J.; Ma, L.F.; Li, D.S. Self-assembly and near-infrared photothermal conversion research of molecular figure-of-eight. J. Solid State Chem. 2022, 313, 123320–123328. [Google Scholar] [CrossRef]
- Dang, L.L.; Zhang, T.T.; Li, T.T.; Chen, T.; Zhao, Y.; Zhao, C.C.; Ma, L.F. Stable Zinc-Based Metal-Organic Framework Photocatalyst for Effective Visible-Light-Driven Hydrogen Production. Molecules 2022, 27, 1917. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Crystallogr. Sect. A Found. Crystallogr. 1990, 46, 467–473. [Google Scholar] [CrossRef]







| Complex | 1 |
|---|---|
| Empirical formula | C76H72N16Ni2O20S2 |
| Formula weight | 1711.03 |
| Temperature/K | 293(2) |
| Crystal system | triclinic |
| Space group | P-1 |
| a/Å | 11.6770(5) |
| b/Å | 18.4276(15) |
| c/Å | 21.3747(11) |
| Volume/Å3 | 4144.5(5) |
| Z | 2 |
| ρcalc/g cm–3 | 1.371 |
| µ/mm–1 | 0.583 |
| F(000)/e | 1776.0 |
| 2θ range for data collection/° | 6.708 to 50.052 |
| Reflections collected | 27223 |
| Independent reflections | 14551 [Rint = 0.0757, Rsigma = 0.1491] |
| Data/restraints/parameters | 14551/12/980 |
| Goodness-of-fit on F2 | 1.002 |
| Δρfin (max/min), e Å–3 | 1.27/–0.63 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0905, wR2 = 0.1580 |
| Final R indexes [all data] | R1 = 0.1581, wR2 = 0.1893 |
| Ni1 | O1 1 | 2.082(5) | Ni1 | O10 3 | 2.083(4) |
| Ni1 | O4 2 | 2.068(5) | Ni1 | O16 4 | 1.987(5) |
| Ni1 | N8 | 2.124(5) | |||
| Ni1 | N9 | 2.109(5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, X.; Dang, L.; Zhang, T.; Chen, T.; Ren, F.; Liu, S. Stable Nickel-Based Metal–Organic Framework Containing Thiophene/Diimidazole Units for Effective Near-Infrared Photothermal Conversion. Catalysts 2022, 12, 777. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12070777
Pei X, Dang L, Zhang T, Chen T, Ren F, Liu S. Stable Nickel-Based Metal–Organic Framework Containing Thiophene/Diimidazole Units for Effective Near-Infrared Photothermal Conversion. Catalysts. 2022; 12(7):777. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12070777
Chicago/Turabian StylePei, Xiangran, Lilong Dang, Tingting Zhang, Tian Chen, Fuxuan Ren, and Shuiren Liu. 2022. "Stable Nickel-Based Metal–Organic Framework Containing Thiophene/Diimidazole Units for Effective Near-Infrared Photothermal Conversion" Catalysts 12, no. 7: 777. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12070777