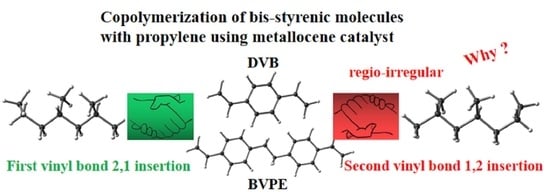
Density Functional Theory Study of the Regioselectivity in Copolymerization of bis-Styrenic Molecules with Propylene Using Zirconocene Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrogen Reactivates the Reaction
2.2. The Second Vinyl Bond of the bis-Styrenic Molecule (DVB and BVPE)
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sturzel, M.; Mihan, S.; Mulhaupt, R. From multisite polymerization catalysis to sustainable materials and all-polyolefin composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef] [PubMed]
- Stanic, S.; Koch, T.; Schmid, K.; Knaus, S.; Archodoulaki, V.M. Improving rheological and mechanical properties of various virgin and recycled polypropylenes by blending with long-chain branched polypropylene. Polymers 2021, 13, 1137. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.T.; Poornima, V.P.; George, S.C.; Kalarikkal, N.; Thomas, S. Enhanced mechanical and thermal performance of multiwalled carbon nanotubes-filled polypropylene/natural rubber thermoplastic elastomers. New J. Chem. 2021, 45, 4963–4976. [Google Scholar] [CrossRef]
- Leone, G.; Palucci, B.; Zanchin, G.; Vignali, A.; Ricci, G.; Bertini, F. Dynamically cross-linked polyolefins via hydrogen bonds: Tough yet soft thermoplastic elastomers with high elastic recovery. ACS Appl. Poly. Mater. 2022, 4, 3770–3778. [Google Scholar] [CrossRef]
- Jasinska-Walc, L.; Bouyahyi, M.; Duchateau, R. Potential of functionalized polyolefins in a sustainable polymer economy: Synthetic strategies and applications. Acc. Chem. Res. 2022, 55, 1985–1996. [Google Scholar] [CrossRef]
- Burkey, A.A.; Fischbach, D.M.; Wentz, C.M.; Beers, K.L.; Sita, L.R. Highly versatile strategy for the production of telechelic polyolefins. ACS Macro Lett. 2022, 11, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Hu, Y.L. Design and synthesis of structurally well-defined functional polyolefins via transition metal-mediated olefin polymerization chemistry. Coord. Chem. Rev. 2006, 250, 47–65. [Google Scholar] [CrossRef]
- Chen, C.L. Designing transition metal catalysts for olefin polymerization and copolymerization: Beyond electronic and steric tuning. Nat. Rev. Chem. 2018, 2, 6–14. [Google Scholar] [CrossRef]
- Gies, A.P.; Zhou, Z.; Sun, L.; Szuromi, E.; Huang, T.; Krasovskiy, A.; Mukhopadhyay, S.; Herceg, E.; Kobylianskii, I.; Shi, Z.; et al. Microstructure characterization of functionalized ethylene/propylene polyolefins. Macromolecules 2022, 55, 2542–2556. [Google Scholar] [CrossRef]
- Wallace, M.A.; Burkey, A.A.; Sita, L.R. Phenyl-terminated polyolefins via living coordinative chain transfer polymerization with ZnPh2 as a chain transfer agent. ACS Catal. 2021, 11, 10170–10178. [Google Scholar] [CrossRef]
- Franssen, N.M.G.; Reek, J.N.H.; de Bruin, B. Synthesis of functional ‘polyolefins’: State of the art and remaining challenges. Chem. Soc. Rev. 2013, 42, 5809–5832. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Gao, H.; Yang, F.; Pan, L.; Wang, B.; Ma, Z.; Li, Y.S. Stereoblock polypropylenes prepared by efficient chain shuttling polymerization of propylene with binary zirconium catalysts and iBu3Al. Chin. J. Poly. Sci. 2020, 38, 1192–1201. [Google Scholar] [CrossRef]
- Huang, J.; Veksha, A.; Chan, W.P.; Lisak, G. Support effects on thermocatalytic pyrolysis-reforming of polyethylene over impregnated ni catalysts. Appl. Catal. A Gen. 2021, 622, 118222. [Google Scholar] [CrossRef]
- Huang, H.; Cao, C.; Niu, H.; Dong, J.Y. Catalytic synthesis of styryl-capped isotactic polypropylenes. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3709–3713. [Google Scholar] [CrossRef]
- Cao, C.; Dong, J.Y.; Chung, T.-C. Hydrogen-assisted unique incorporation of 1,4-divinylbenzene in copolymerization with propylene using an isospecific zirconocene catalyst. Macromol. Rapid Commun. 2005, 26, 1936–1941. [Google Scholar] [CrossRef]
- Zhang, C.G.; Yu, S.Y.; Zhang, L.; Li, H.; Wang, Z.X. DFT mechanistic study of the H2-assisted chain transfer copolymerization of propylene and p-methylstyrene catalyzed by zirconocene complex. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 576–585. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Benchmark energetic data in a model system for grubbs II metathesis catalysis and their use for the development, assessment, and validation of electronic structure methods. J. Chem. Theory Comput. 2009, 5, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Simon, S.; Duran, M.; Dannenberg, J.J. How does basis set superposition error change the potential surfaces for hydrogen bonded dimers? J. Chem. Phys. 1996, 105, 11024–11031. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Dang, Y.; Qu, S.; Wang, Z.-X.; Wang, X. A computational mechanistic study of an unprecedented heck-type relay reaction: Insight into the origins of regio- and enantioselectivities. J. Am. Chem. Soc. 2014, 136, 986–998. [Google Scholar] [CrossRef]
- Deng, L.; Fu, Y.; Lee, S.Y.; Wang, C.; Liu, P.; Dong, G. Kinetic resolution via Rh-catalyzed C-C activation of cyclobutanones at room temperature. J. Am. Chem. Soc. 2019, 141, 16260–16265. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Qu, L.-B.; Houk, K.N.; Lan, Y. Origin of regiochemical control in Rh(III)/Rh(V)-catalyzed reactions of unsaturated oximes and alkenes to form pyrdines. ACS Catal. 2019, 9, 7154–7165. [Google Scholar] [CrossRef]
- Palani, V.; Hugelshofer, C.L.; Kevlishvili, I.; Liu, P.; Sarpong, R. A short synthesis of delavatine a unveils new insights into site-selective cross-coupling of 3,5-dibromo-2-pyrone. J. Am. Chem. Soc. 2019, 141, 2652–2660. [Google Scholar] [CrossRef]
- Qi, X.; Kohler, D.G.; Hull, K.L.; Liu, P. Energy decomposition analyses reveal the origins of catalyst and nucleophile effects on regioselectivity in nucleopalladation of alkenes. J. Am. Chem. Soc. 2019, 141, 11892–11904. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, X.Y.; Wang, Z.X. Insight into the selective methylene oxidation catalyzed by Mn(CF3-PDP)(SbF6)2/H2O2/CH2ClCO2H) system: A DFT mechanistic study. Org. Lett. 2021, 23, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, Y.; Guo, J.; Hu, W.; Wang, Z.X. DFT mechanistic account for the site selectivity of electron-rich C(sp3)–H bond in the manganese-catalyzed aminations. Org. Lett. 2020, 22, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Lu, Y.; Lu, G.; Wang, Z.X. Density functional theory mechanistic study of Ni-catalyzed reductive alkyne–alkyne cyclodimerization: Oxidative cyclization versus outer-sphere proton transfer. Org. Lett. 2020, 22, 2454–2459. [Google Scholar] [CrossRef]
- Yu, S.Y.; Ren, P.; Zheng, H.-M.; Zhang, C.G. Copolymerization mechanisms of propylene and norbornadiene catalyzed by zirconocene complexes: A density functional theory study. Bull. Korean Chem. Soc. 2018, 39, 381–385. [Google Scholar] [CrossRef]
- Zhang, C.G.; Zhang, L.; Li, H.; Yu, S.Y.; Wang, Z.X. Differences between insertions of ethylene into metallocene and non-metallocene ethylene polymerization catalysts. J. Phys. Org. Chem. 2013, 26, 70–76. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.-W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q.X. Realization of conceptual density functional theory and information-theoretic approach in Multiwfn program. In Conceptual Density Functional Theory; WILEY-VCH GmbH: Weinheim, Germany, 2022; pp. 631–647. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness-companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Von Szentpaly, L.; Liu, S.B. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. a theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Fu, R.; Lu, T.; Chen, F.-W. Comparing methods for predicting the reactive site of electrophilic substitution. Acta Phys.-Chim. Sin. 2014, 30, 628–639. [Google Scholar]










| WBI | QNBO (e) | |||||
|---|---|---|---|---|---|---|
| B (Zr-C1) | B (H1-H2) | B (Zr-H1) | B (C1-H2) | Zr | H2 | |
| [Zr]-DVB[BVPE]-PP1 + H2 | 0.609 [0.619] | 1.0 [1.0] | 1.358 [1.362] | 0.000 [0.0] | ||
| TS1 | 0.563 [0.571] | 0.923 [0.942] | 0.056 [0.040] | 0.001 [0.001] | 1.375 [1.403] | 0.011 [0.005] |
| IM1 | 0.590 [0.596] | 0.713 [0.712] | 0.236 [0.236] | 0.049 [0.050] | 0.877 [0.879] | 0.100 [0.100] |
| TS2 | 0.504 [0.509] | 0.496 [0.496] | 0.416 [0.417] | 0.239 [0.239] | 0.815 [0.815] | 0.132 [0.132] |
| IM2 | 0.003 [0.003] | 0.000 [0.000] | 0.880 [0.882] | 0.892 [0.895] | 1.112 [1.089] | 0.261 [0.259] |
| DVB[BVPE]-PP1 + [Zr]-H | 0.877 [0.877] | 0.902 [0.912] | 1.311 [1.311] | 0.245 [0.240] | ||
| WBI | QNBO (e) | |||||
|---|---|---|---|---|---|---|
| B (Zr-H1) | B (C2-C3) | B (Zr-C2) | B (C3-H1) | Zr | C2 | |
| DVB[BVPE]-PP1+ [Zr]-H | 0.877 [0.877] | 1.897 [1.898] | 1.311 [1.311] | −0.421 [−0.419] | ||
| IM31,2 | 0.889 [0.889] | 1.595 [1.598] | 0.345 [0.342] | 0.006 [0.005] | 1.095 [1.095] | −0.643 [−0.639] |
| TS31,2 | 0.604 [0.604] | 1.392 [1.145] | 0.529 [0.530] | 0.283 [0.291] | 1.148 [1.149] | −0.703 [−0.704] |
| PR1,2 | 0.144 [0.143] | 1.024 [1.023] | 0.788 [0.788] | 0.782 [0.784] | 1.319 [1.320] | −0.806 [−0.806] |
| B (Zr-H1) | B (C2-C3) | B (Zr-C3) | B (C3-H1) | Zr | C3 | |
| IM32,1 | 0.817 [0.819] | 1.693 [1.697] | 0.235 [0.232] | 0.067 [0.065] | 0.940 [0.948] | −0.302 [−0.301] |
| TS32,1 | 0.636 [0.634] | 1.497 [1.495] | 0.383 [0.385] | 0.261 [0.263] | 0.890 [0.888] | −0.352 [−0.353] |
| PR2,1 | 0.181 [0.182] | 1.081 [1.083] | 0.650 [0.647] | 0.747 [0.745] | 1.043 [1.037] | −0.469 [−0.465] |
| η a | μ b | ω c | NNud | ωlocale | NNu-localf | f+g | f−h | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Zr | C2 | C3 | Zr | C2 | C3 | |||||
| [Zr]-DVB-PP1 | 4.594 | −6.838 | 5.088 | 1.064 | 0.842 | 0.040 | 0.012 | 0.166 | 0.038 | 0.011 |
| [Zr]-[BVPE]-PP1 | 4.295 | −6.637 | 5.128 | 1.619 | 0.854 | 0.031 | 0.028 | 0.167 | 0.075 | 0.039 |
| [Zr]-H | 4.945 | −7.212 | 5.260 | 0.588 | 1.298 | 0.247 | ||||
| DVB-PP1 | 8.748 | −3.271 | 0.611 | 3.311 | 0.473 | 0.221 | 0.143 | 0.067 | ||
| [BVPE]-PP1 | 8.145 | −3.247 | 0.647 | 3.264 | 0.308 | 0.140 | 0.094 | 0.043 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.-Y.; Peng, X.; Wang, F.; Cao, J.; Wang, F.; Zhang, C.-G. Density Functional Theory Study of the Regioselectivity in Copolymerization of bis-Styrenic Molecules with Propylene Using Zirconocene Catalyst. Catalysts 2022, 12, 1039. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12091039
Yu S-Y, Peng X, Wang F, Cao J, Wang F, Zhang C-G. Density Functional Theory Study of the Regioselectivity in Copolymerization of bis-Styrenic Molecules with Propylene Using Zirconocene Catalyst. Catalysts. 2022; 12(9):1039. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12091039
Chicago/Turabian StyleYu, Shu-Yuan, Xiaoxia Peng, Fuping Wang, Jian Cao, Fei Wang, and Cheng-Gen Zhang. 2022. "Density Functional Theory Study of the Regioselectivity in Copolymerization of bis-Styrenic Molecules with Propylene Using Zirconocene Catalyst" Catalysts 12, no. 9: 1039. https://0-doi-org.brum.beds.ac.uk/10.3390/catal12091039