Fabrication of Porous Hydrophilic CN/PANI Heterojunction Film for High-Efficiency Photocatalytic H2 Evolution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Structure Characterization
2.2. Photocatalytic Performance Evaluation
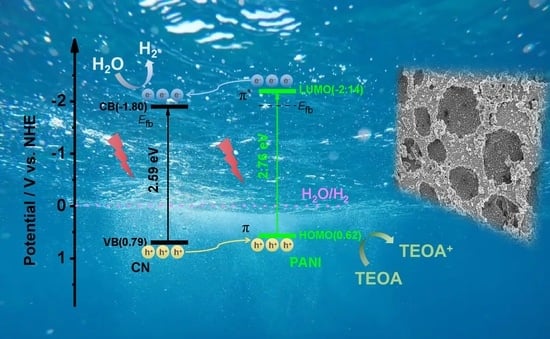
2.3. Mechanism Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Photocatalysts Preparation Procedure
3.2.1. Synthesis of CN
3.2.2. Synthesis of CN/PANI Composites and CN/PANI@PCL Film
3.3. Characterizations
3.4. Photoelectrochemical Tests
3.5. Evaluation of Photocatalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Liu, X.L.; He, L.; Zhang, Y.X.; Hu, Z.-Y.; Tian, G.; Cheng, X.; Wu, S.-M.; Li, Y.-Z.; Yang, X.-H.; et al. Spatial heterojunction in nanostructured TiO2 and its cascade effect for efficient photocatalysis. Nano Lett. 2020, 20, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Takanabe, K.; Maeda, K.; Domen, K.; Epping, J.D.; Fu, X.; Antonietti, M.; Wang, X. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem. Int. Ed. Engl. 2010, 49, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Y.X.; He, L.; Wang, L.Y.; Liu, X.L.; Liu, J.-W.; Li, Y.-Z.; Tian, G.; Zhao, H.; Yan, X.-H.; et al. Interfacial co-existence of oxygen and titanium vacancies in nanostructured Tio2 for enhancement of carrier transport. Nanoscale 2020, 12, 8364–8370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lan, Z.A.; Lin, L.; Lin, S.; Wang, X. Overall water splitting by Pt/g-C3N4 photocatalysts without using sacrificial agents. Chem. Sci. 2016, 7, 3062–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, K.A.; Harris, D.F.; Wilker, M.B.; Rasmussen, A.; Khadka, N.; Hamby, H.; Keable, S.; Dukovic, G.; Peters, J.W.; Seefeldt, L.C.; et al. Light-driven dinitrogen reduction catalyzed by a CdS:nitrogenase MoFe protein biohybrid. Science 2016, 352, 448–450. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Yan, T.; Hu, Z.; Jelle, A.A.; Meira, D.M.; Duchesne, P.N.; Loh, J.Y.Y.; Qiu, C.; Storey, E.E.; et al. Black indium oxide a photothermal CO2 hydrogenation catalyst. Nat. Commun. 2020, 11, 2432–2440. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, X.; Chen, L.; Xiong, Y.; Xu, H. Van Der Waals heterostructures comprised of ultrathin polymer nanosheets for efficient Z-scheme overall water splitting. Angew. Chem. Int. Ed. Engl. 2018, 57, 3454–3458. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Barrio, J.; Volokh, M.; Shalom, M. Polymeric carbon nitrides and related metal-free materials for energy and environmental applications. J. Mater. Chem. A 2020, 8, 11075–11116. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Rodrigues, M.T.F.; et al. High efficiency photocatalytic water splitting using 2D α-Fe2O3/g-C3N4 Z-scheme catalysts. Adv. Energy Mater. 2017, 7, 1700025–1700032. [Google Scholar] [CrossRef]
- Fu, Y.; Ren, Z.; Wu, J.; Li, Y.; Liu, W.; Li, P.; Xing, L.; Ma, J.; Wang, H.; Xue, X. Direct Z-scheme heterojunction of ZnO/MoS2 nanoarrays realized by flowing-induced piezoelectric field for enhanced sunlight photocatalytic performances. Appl. Catal. B Environ. 2021, 285, 119785–119795. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, D.; Sun, Y.; Sheng, X.; Zhao, J.; Guo, J.; Zhou, B. g-C3N4 and polyaniline-co-modified TiO2 nanotube arrays for significantly enhanced photocatalytic degradation of tetrabromobisphenol A under visible light. Chemosphere 2020, 252, 126468–126475. [Google Scholar] [CrossRef]
- Wu, H.; Chang, C.; Lu, D.; Maeda, K.; Hu, C. Synergistic effect of hydrochloric acid and phytic acid doping on polyaniline-coupled g-C3N4 nanosheets for photocatalytic Cr(VI) reduction and dye degradation. ACS Appl. Mater. Interfaces 2019, 11, 35702–35712. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J. In situ synthesis and enhanced visible light photocatalytic activities of novel PANI-g-C3N4 composite photocatalysts. J. Mater. Chem. 2012, 22, 11843–11850. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, L.; Zeng, M.; Li, J.; Xu, J.; Wang, X. Hierarchical nanocomposites of polyaniline nanorods arrays on graphitic carbon nitride sheets with synergistic effect for photocatalysis. Catal. Today 2014, 224, 114–121. [Google Scholar] [CrossRef]
- Zhang, M.; Bao, Y.; Hou, L.; Gao, K.; Yang, Y. Will the photocatalytic ceramic membrane be the solution for the next generation of photocatalysis?—A comprehensive comparison between g-C3N4 powder and g-C3N4 modified ceramic membrane. Sep. Purif. Technol. 2023, 305, 122440–122451. [Google Scholar] [CrossRef]
- Jia, C.; Yang, L.; Zhang, Y.; Zhang, X.; Xiao, K.; Xu, J.; Liu, J. Graphitic carbon nitride films: Emerging paradigm for versatile applications. ACS Appl. Mater. Interfaces 2020, 12, 53571–53591. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Qin, N.; Wu, J.; Yuan, B.; Kang, Z.; Bao, D. BaTiO3 nanosheets and caps grown on TiO2 nanorod arrays as thin-film catalysts for piezocatalytic applications. ACS Appl. Mater. Interfaces 2020, 12, 14005–14015. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, J.; Guo, Z. Polyaniline coated membranes for effective separation of oil-in-water emulsions. J. Colloid Interface Sci. 2016, 467, 261–270. [Google Scholar] [CrossRef]
- Yang, X.; Bian, X.; Yu, W.; Wang, Q.; Huo, X.; Teng, S. Organosilica-assisted superhydrophilic oxygen doped graphitic carbon nitride for improved photocatalytic H2 evolution. Int. J. Hydrog. Energy 2022, 47, 34444–34454. [Google Scholar] [CrossRef]
- Naciri, Y.; Hsini, A.; Bouziani, A.; Tanji, K.; El Ibrahimi, B.; Ghazzal, M.N.; Bakiz, B.; Albourine, A.; Benlhachemi, A.; Navío, J.A.; et al. Z-scheme WO3/PANI Heterojunctions with enhanced photocatalytic activity under visible light: A depth experimental and DFT studies. Chemosphere 2022, 292, 133468–133481. [Google Scholar] [CrossRef]
- Qin, N.; Pan, A.; Yuan, J.; Ke, F.; Wu, X.; Zhu, J.; Liu, J.; Zhu, J. One-step construction of a hollow Au@bimetal-organic framework core-shell catalytic nanoreactor for selective Alcohol oxidation reaction. ACS Appl. Mater. Interfaces 2021, 13, 12463–12471. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098–112106. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D porous zinc-organic framework platform for loading of 5-fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Jin, J.C.; Wu, X.R.; Luo, Z.D.; Deng, F.Y.; Wu, X.; Luo, Z. Luminescent sensing and photocatalytic degradation properties of an uncommon (4, 5, 5)-connected 3D MOF based on 3, 5-di (3′, 5′-dicarboxylphenyl) benzoic acid. CrystEngComm 2017, 19, 4368–4377. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, Y.; Yang, X. High efficiency photocatalytic CO2 reduction realized by Ca2+ and HDMP group co-modified graphitic carbon nitride. Int. J. Hydrog. Energy 2021, 46, 32893–32903. [Google Scholar] [CrossRef]
- Poulain, M. Significantly improving the performance and dispersion morphology of porous g-C3N4/PANI composites by an interfacial polymerization method. e-Polymers 2015, 15, 95–101. [Google Scholar]
- Yu, C.F.; Tan, L.; Shen, S.J.; Fang, M.H.; Yang, L.; Fu, X.; Dong, S.; Sun, J. In situ preparation of g-C3N4/polyaniline hybrid composites with enhanced visible-light photocatalytic performance. J. Environ. Sci. 2021, 104, 317–325. [Google Scholar] [CrossRef]
- Li, Y.M.; Zhong, J.B.; Li, J.Z. Reinforced photocatalytic H2 generation behavior of S-scheme NiO/g-C3N4 heterojunction photocatalysts with enriched nitrogen vacancies. Opt. Mater. 2023, 135, 113296–113304. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, Y.; Li, X.; Jing, Y.; Zhang, Z.; Gao, Y.; Liu, J.; Tan, H.; Cai, X.; Cai, J. Ultrathin g-PAN/PANI-encapsulated Cu nanoparticles decorated on SrTiO3 with high stability as an efficient photocatalyst for the H2 evolution and degradation of 4-nitrophenol under visible-light irradiation. Catal. Sci. Technol. 2022, 12, 2482–2489. [Google Scholar] [CrossRef]
- Li, T.; Cui, J.D.; Gao, L.M.; Lin, Y.-Z.; Li, R.; Xie, H.; Zhang, Y.; Li, K. Competitive self-assembly of PANI confined MoS2 boosting the photocatalytic activity of the graphitic carbon nitride. ACS Sustain. Chem. Eng. 2020, 8, 13352–13361. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, A.; Cheng, T.; Pan, J.; Song, J.; Zhang, L.; Guan, R.; Xi, X.; Zhang, J. 2D/2D S-scheme heterojunction with a covalent organic framework and g-C3N4 nanosheets for highly efficient photocatalytic H2 evolution. Chin. J. Catal. 2022, 43, 2592–2605. [Google Scholar] [CrossRef]
- Sk, S.; Vennapoosa, C.S.; Tiwari, A.; Abraham, B.M.; Pal, U. Polyaniline encapsulated Ti-MOF/CoS for efficient photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2022, 47, 33955–33965. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Z.; Zhang, X.; Han, Y.; Xue, Z.; Xie, T.; Yang, W. The effect of indium doping on the hydrogen evolution performance of g-C3N4 based photocatalysts. New J. Chem. 2021, 45, 544–550. [Google Scholar] [CrossRef]
- Zhu, H.; Cai, S.; Liao, G.; Gao, Z.F.; Min, X.; Huang, Y.; Jin, S.; Xia, F. Recent advances in photocatalysis based on bioinspired superwettabilities. ACS Catal. 2021, 11, 14751–14771. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Sun, S.; Ren, J.; Zhou, L.; Zhang, L. Efficient visible light-induced photocatalytic degradation of contaminant by spindle-like PANI/BiVO4. J. Phys. Chem. C 2009, 113, 20228–20233. [Google Scholar] [CrossRef]
- Hung, S.F.; Xiao, F.X.; Hsu, Y.Y.; Suen, Y.-Y.; Yang, N.-T.; Chen, H.-B.; Liu, H.M.; Chen, B.H.M. Iridium oxide-assisted plasmon-induced hot carriers: Improvement on kinetics and thermodynamics of hot carriers. Adv. Energy Mater. 2016, 6, 1501339–1501351. [Google Scholar] [CrossRef]
- Moura, N.K.d.; Siqueira, I.A.W.B.; Machado, J.P.d.B.; Kido, H.W.; Avanzi, I.R.; Rennó, A.C.M.; Trichês, E.d.S.; Passador, F.R. Production and characterization of porous polymeric membranes of PLA/PCL blends with the addition of hydroxyapatite. J. Compos. Sci. 2019, 3, 45. [Google Scholar] [CrossRef]








| Photocatalyst | BET Surface Area (m2·g−1) | Pore Size (nm) | Pore Volume (cm3·g−1) |
|---|---|---|---|
| CN | 9.47 | 9.6 | 0.057 |
| PANI | 5.75 | 15.9 | 0.045 |
| CN/PANI heterojunction film | 137.87 | 9.4 | 0.177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, Y.; Deng, J.; Huo, X.; Wang, Y.; Jia, R. Fabrication of Porous Hydrophilic CN/PANI Heterojunction Film for High-Efficiency Photocatalytic H2 Evolution. Catalysts 2023, 13, 139. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13010139
Yang X, Zhang Y, Deng J, Huo X, Wang Y, Jia R. Fabrication of Porous Hydrophilic CN/PANI Heterojunction Film for High-Efficiency Photocatalytic H2 Evolution. Catalysts. 2023; 13(1):139. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13010139
Chicago/Turabian StyleYang, Xiaohang, Yulin Zhang, Jiayuan Deng, Xuyang Huo, Yanling Wang, and Ruokun Jia. 2023. "Fabrication of Porous Hydrophilic CN/PANI Heterojunction Film for High-Efficiency Photocatalytic H2 Evolution" Catalysts 13, no. 1: 139. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13010139