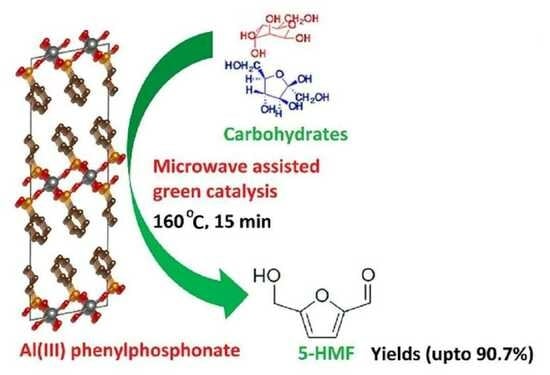
Organically Functionalized Porous Aluminum Phosphonate for Efficient Synthesis of 5-Hydroxymethylfurfural from Carbohydrates
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Catalytic Properties
3.1.1. Catalysis with Ph-ALPO-2
3.1.2. Catalysis with Ph-ALPO-1
3.2. Role of Template in Synthesizing Phenyl Aluminophosphate Catalyst
3.3. Comparision of Ph-ALPO-2 with Other Catalysts in the Literature
4. Materials and Methods
4.1. Chemicals
4.2. Instrumentation
4.3. Synthesis of Ph-ALPO-1
4.4. Synthesis of Ph-ALPO-2
4.5. Extraction of Template from Ph-ALPO-2
4.6. Activation of Catalyst
4.7. 5-HMF Synthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.T.; Romani, A.; Domingues, L. Whole Cell Biocatalysis of 5-Hydroxymethylfurfural for Sustainable Biorefineries. Catalysts 2022, 12, 202. [Google Scholar] [CrossRef]
- Barta, K.; Ford, P.C. Catalytic Conversion of Nonfood Woody Biomass Solids to Organic Liquids. Acc. Chem. Res. 2014, 47, 1503–1512. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar]
- Liu, Y.Z.; Chen, W.S.; Xia, Q.Q.; Guo, B.T.; Wang, Q.W.; Liu, S.X.; Liu, Y.X.; Li, J.; Yu, H.P. Efficient Cleavage of Lignin-Carbohydrate Complexes and Ultrafast Extraction of Lignin Oligomers from Wood Biomass by Microwave-Assisted Treatment with Deep Eutectic Solvent. ChemSusChem 2017, 10, 1692–1700. [Google Scholar] [CrossRef]
- Chheda, J.N.; Roman-Leshkov, Y.; Dumesic, J.A. Production of 5-Hydroxymethylfurfural and Furfural by Dehydration of Biomass-Derived Mono- and Poly-Saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Osatiashtiani, A.; Lee, A.F.; Brown, D.R.; Melero, J.A.; Morales, G.; Wilson, K. Bifunctional SO4/ZrO2 Catalysts for 5-Hydroxymethylfufural (5-HMF) Production from Glucose. Catal. Sci. Technol. 2014, 4, 333–342. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Solanki, B.S.; Rode, C.V. Selective Hydrogenation of 5-HMF to 2,5-DMF Over a Magnetically Recoverable Non-Noble Metal Catalyst. Green Chem. 2019, 21, 6390–6406. [Google Scholar]
- Cortez-Elizalde, J.; Córdova-Pérez, G.E.; Silahua-Pavón, A.A.; Pérez-Vidal, H.; Cervantes-Uribe, A.; Cordero-García, A.; Arévalo-Pérez, J.C.; Becerril-Altamirano, N.L.; Castillo-Gallegos, N.C.; Lunagómez-Rocha, M.A.; et al. 2,5-Dimethylfuran Production by Catalytic Hydrogenation of 5-Hydroxymethylfurfural Using Ni Supported on Al2O3-TiO2-ZrO2 Prepared by Sol-Gel Method: The Effect of Hydrogen Donors. Molecules 2022, 27, 4187. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.L.; Ma, Y.; Li, Y.D. Biomass into Chemicals: Conversion of Sugars to Furan Derivatives by Catalytic Processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Tempelman, C.H.L.; Oozeerally, R.; Degirmenci, V. Heterogeneous Catalysts for the Conversion of Glucose into 5-Hydroxymethyl Furfural. Catalysts 2021, 11, 861. [Google Scholar] [CrossRef]
- Bains, R.; Kumar, A.; Chauhan, A.S.; Das, P. Dimethyl carbonate solvent assisted efficient conversion of lignocel lulosic biomass to 5-hydroxymethylfurfural and furfural. Renew. Energy 2022, 197, 237–243. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, J.; Bhaumik, A. Sulfonated Porous Polymeric Nanofibers as an Efficient Solid Acid Catalyst for the Production of 5-Hydroxymethylfurfural from Biomass. ChemCatChem 2015, 7, 3570–3578. [Google Scholar] [CrossRef]
- Yin, Y.; Ma, C.H.; Li, W.; Luo, S.; Zhang, Z.S.; Liu, S.X. Insights into Shape Selectivity and Acidity Control in NiO-Loaded Mesoporous SBA-15 Nanoreactors for Catalytic Conversion of Cellulose to 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2022, 10, 17081–17093. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhao, B.W.; Das, S.; Degirmenci, V.; Walton, R.L. Tuning the Hydrophobicity and Lewis Acidity of UiO-66-NO2 with Decanoic Acid as Modulator to Optimise Conversion of Glucose to 5-Hydroxymethylfurfural. Catalysts 2022, 12, 1502. [Google Scholar] [CrossRef]
- Elhamifar, D.; Nasr-Esfahani, M.; Karimi, B.; Moshkelgosha, R.; Shábani, A. Ionic Liquid and Sulfonic Acid Based Bifunctional Periodic Mesoporous Organosilica (BPMO-IL-SO3H) as a Highly Efficient and Reusable Nanocatalyst for the Biginelli Reaction. ChemCatChem 2014, 6, 2593–2599. [Google Scholar]
- Herbst, A.; Janiak, C. MOF Catalysts in Biomass Upgrading Towards Value-Added Fine Chemicals. CrystEngComm 2017, 19, 4092–4117. [Google Scholar]
- Chongdar, S.; Bhattacharjee, S.; Bhanja, P.; Bhaumik, A. Porous organic–inorganic hybrid materials for catalysis, energy and environmental applications. Chem. Commun. 2022, 58, 3429–3460. [Google Scholar]
- Lewis, D.W.; Willock, D.J.; Catlow, C.R.A.; Thomas, J.M.; Hutchings, G.J. De Novo Design of Structure-Directing Agents for the Synthesis of Microporous Solids. Nature 1996, 382, 604–606. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The Hydrothermal Synthesis of Zeolites: Precursors, Intermediates and Reaction Mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar]
- Jiang, J.X.; Yu, J.H.; Corma, A. Extra-Large-Pore Zeolites: Bridging the Gap between Micro and Mesoporous Structures. Angew. Chem. Int. Ed. 2010, 49, 3120–3145. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.T.; Lok, B.M.; Messina, C.A.; Cannan, T.R.; Flanigen, E.M. Aluminophosphate Molecular Sieves: A New Class of Microporous Crystalline Inorganic Solids. J. Am. Chem. Soc. 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- Davis, M.E.; Saldarriaga, C.; Montes, C.; Garces, J.; Crowder, C. A Molecular-Sieve With 18-Membered Rings. Nature 1988, 331, 698–699. [Google Scholar] [CrossRef]
- Mizoshita, N.; Tani, T.; Inagaki, S. Syntheses, Properties and Applications of Periodic Mesoporous Organosilicas Prepared from Bridged Organosilane Precursors. Chem. Soc. Rev. 2011, 40, 789–800. [Google Scholar] [CrossRef]
- Bhanja, P.; Na, J.; Jing, T.; Lin, J.J.; Wakihara, T.; Bhaumik, A.; Yamauchi, Y. Nanoarchitectured Metal Phosphates and Phosphonates: A New Material Horizon toward Emerging Applications. Chem. Mater. 2019, 31, 5343–5362. [Google Scholar] [CrossRef]
- Gagnon, K.J.; Perry, H.P.; Clearfield, A. Conventional and Unconventional Metal-Organic Frameworks Based on Phosphonate Ligands: MOFs and UMOFs. Chem. Rev. 2012, 112, 1034–1054. [Google Scholar] [CrossRef]
- Li, J.Y.; Qi, M.; Kong, J.; Wang, J.Z.; Yan, Y.; Huo, W.F.; Yu, J.H.; Xu, R.R.; Xu, Y. Computational Prediction of the Formation of Microporous Aluminophosphates with Desired Structural Features. Microporous Mesoporous Mater. 2010, 129, 251–255. [Google Scholar] [CrossRef]
- Konstantin, I.; Galkin, K.I.; Krivodaeva, E.A.; Romashov, L.V.; Zalesskiy, S.S.; Kachala, V.V.; Burykina, J.V.; Ananikov, V.P. Critical Influence of 5-Hydroxymethylfurfural Aging and Decomposition on the Utility of Biomass Conversion in Organic Synthesis. Angew. Chem. Int. Ed. 2016, 55, 8338–8342. [Google Scholar]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A Kit of Tools for Phasing Crystal Structures from Powder Data. J. Appl. Cryst. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Chowdhury, A.; Bhattacharjee, S.; Chatterjee, R.; Bhaumik, A. A New Nitrogen Rich Porous Organic Polymer for Ultra-High CO2 Uptake and as An Excellent Organocatalyst for CO2 Fixation Reactions. J. CO2 Util. 2022, 65, 102236. [Google Scholar] [CrossRef]
- Shahangi, F.; Chermahini, A.N.; Saraji, M. Dehydration of fructose and glucose to 5-hydroxymethylfurfural over Al-KCC-1 silica. J. Energy Chem. 2018, 27, 769–780. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, X.; Huang, C.; Li, Y.; Chen, B. Niobium phosphotungstates: Excellent solid acid catalysts for the dehydration of fructose to 5-hydroxymethylfurfural under mild conditions. RSC Adv. 2018, 8, 32423–32433. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.W.; Su, A.X.; Li, X.M. Preparation of Aluminosilicate Mesoporous Catalyst and its Application for Production 5-Hydroxymethyl Furfural Dehydration from Fructose. Adv. Res. Mater. 2011, 396–398, 1190–1193. [Google Scholar] [CrossRef]
- Dutta, A.; Gupta, D.; Patra, A.K.; Saha, B.; Bhaumik, A. Synthesis of 5-Hydroxymethylfurural from Carbohydrates using Large-Pore Mesoporous Tin Phosphate. ChemSusChem 2014, 7, 925–933. [Google Scholar] [CrossRef]
- Crisci, A.J.; Tucker, M.H.; Lee, M.Y.; Jang, S.G.; Dumesic, J.A.; Scott, S.L. Acid-Functionalized SBA-15-Type Silica Catalysts for Carbohydrate Dehydration. ACS Catal. 2011, 1, 719–728. [Google Scholar] [CrossRef]
- Ghosh, A.; Chowdhury, B.; Bhaumik, A. Synthesis of Hollow Mesoporous Silica Nanospheroids with O/W Emulsion and Al(III) Incorporation and Its Catalytic Application. Catalysts 2023, 13, 354. [Google Scholar] [CrossRef]





| Sl. No. | Substrate Amount (mg) | Catalyst Amount (mg) | Temperature (°C) | Time (min.) | Yield (%) |
|---|---|---|---|---|---|
| 1. | 25.5 | 2 | 150 | 20 | 42.8 |
| 2. | 25.5 | 4 | 150 | 20 | 56.04 |
| 3. | 25.5 | 6 | 150 | 20 | 68.45 |
| 4. | 25.5 | 8 | 150 | 20 | 63.92 |
| 5. | 25.5 | 6 | 160 | 20 | 83.3 |
| 6. | 25.5 | 6 | 170 | 20 | 76.09 |
| 7. | 25.5 | 6 | 160 | 5 | 59.03 |
| 8. | 25.5 | 6 | 160 | 10 | 90.08 |
| 9. | 25.5 | 6 | 160 | 15 | 90.7 |
| 10. | 25.5 | 6 | 160 | 25 | 77.2 |
| 11. | 25.5 | 6 | 160 | 30 | 69.9 |
| Catalyst | Temp. (°C) | Solvent | Time (min.) | Substrate | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Al-KCC-1 | 162 | DMSO | 60 | Fructose | 92.9 | [33] |
| NBPW-01 | 80 | DMSO | 180 | Fructose | 89 | [34] |
| NBPW-06 | 80 | DMSO | 90 | Fructose | 96.7 | [35] |
| Al-MCM-41 | 170 | H2O/MIBK | 75 | Fructose | 42.3 | [36] |
| LPSnP-1 | 120 | H2O/MIBK: 2-butanol | 20 | Fructose | 77 | [36] |
| TESAS-SBA-15 | 130 | H2O/MIBK: 2-butanol | 141 | Fructose | 71 | [37] |
| PHMS-2 | 160 | DMSO | 25 | Fructose | 83.7 | [38] |
| Ph-ALPO-1 | 160 | DMSO | 15 | Fructose | 74.6 | This work |
| Ph-ALPO-2 | 160 | DMSO | 15 | Fructose | 90.7 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitra, R.; Malakar, B.; Bhaumik, A. Organically Functionalized Porous Aluminum Phosphonate for Efficient Synthesis of 5-Hydroxymethylfurfural from Carbohydrates. Catalysts 2023, 13, 1449. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13111449
Mitra R, Malakar B, Bhaumik A. Organically Functionalized Porous Aluminum Phosphonate for Efficient Synthesis of 5-Hydroxymethylfurfural from Carbohydrates. Catalysts. 2023; 13(11):1449. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13111449
Chicago/Turabian StyleMitra, Riddhi, Bhabani Malakar, and Asim Bhaumik. 2023. "Organically Functionalized Porous Aluminum Phosphonate for Efficient Synthesis of 5-Hydroxymethylfurfural from Carbohydrates" Catalysts 13, no. 11: 1449. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13111449