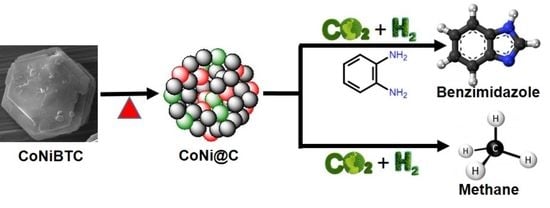
Bimetallic Metal-Organic Framework Derived Nanocatalyst for CO2 Fixation through Benzimidazole Formation and Methanation of CO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysis
Synthesis of Benzimidazoles
2.2. Methanation of CO2
3. Experimental
3.1. Materials and General Methods
3.1.1. Chemicals used in This Work
3.1.2. Instrumentation
3.2. Synthesis of MOFs
3.2.1. Synthesis of CoBTC
3.2.2. Synthesis of NiBTC
3.2.3. Synthesis of CoNiBTC
3.2.4. Synthesis of CoNiBTC-1
3.2.5. Synthesis of CoNiBTC-2
3.2.6. Synthesis of CoNi@C
3.2.7. Synthesis of CoNi@C-1
3.2.8. Synthesis of CoNi@C-2
3.2.9. Synthesis of Co@C
3.2.10. Synthesis of Ni@C
3.3. Catalysis of Benzimidazole Synthesis
3.4. Catalysis of CO2 Methanation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younas, M.; Loong, L.L.; Bashir, M.J.K.; Nadeem, H.; Shehzad, A.; Sethupathi, S. Recent Advancements, Fundamental Challenges, and Opportunities in Catalytic Methanation of CO2. Energy Fuels 2016, 30, 8815–8831. [Google Scholar] [CrossRef]
- Mardani, A.; Streimikiene, D.; Cavallaro, F.; Loganathan, N.; Khoshnoudi, M. Carbon dioxide (CO2) emissions and economic growth: A systematic review of two decades of research from 1995 to 2017. Sci. Total. Environ. 2019, 649, 31–49. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Younas, M.; Sohail, M.; Leong, L.K.; Bashir, M.J.K.; Sumathi, S. Feasibility of CO2 adsorption by solid adsorbents: A review on low-temperature systems. Int. J. Environ. Sci. Technol. 2016, 13, 1839–1860. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Imidazolium Ionic Liquids, Imidazolylidene Heterocyclic Carbenes, and Zeolitic Imidazolate Frameworks for CO2 Capture and Photochemical Reduction. Angew. Chem. Int. Ed. 2016, 55, 2308–2320. [Google Scholar] [CrossRef]
- Listorti, A.; Durrant, J.; Barber, J. Solar to fuel. Nat. Mater. 2009, 8, 929–930. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Lou, X.W. Rationally designed hierarchical N-doped carbon@NiCo2O4 double-shelled nanoboxes for enhanced visible light CO2 reduction. Energy Environ. Sci. 2018, 11, 306–310. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Mazari, S.A.; Hossain, N.; Basirun, W.J.; Mubarak, N.M.; Abro, R.; Sabzoi, N.; Shah, A. An overview of catalytic conversion of CO2 into fuels and chemicals using metal organic frameworks. Process Saf. Environ. Prot. 2021, 149, 67–92. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, K.; Gao, R.; Zhang, L.; Wang, L.; Zhang, C. Research on the Growth Mechanism of PM2.5 in Central and Eastern China during Autumn and Winter from 2013–2020. Atmosphere 2022, 13, 1–38. [Google Scholar]
- Helal, A.; Alahmari, F.; Usman, M.; Yamani, Z.H. Chalcopyrite UiO-67 Metal-Organic Framework Composite for CO2 Fixation as Cyclic Carbonates. J. Environ. Chem. Eng. 2022, 10, 108061–108068. [Google Scholar] [CrossRef]
- Helal, A.; Shah, S.S.; Usman, M.; Khan, M.Y.; Aziz, M.A.; Rahman, M.M. Potential Applications of Nickel-Based Metal-Organic Frameworks and their Derivatives. Chem. Rec. 2022, e202200055. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal-organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045–17069. [Google Scholar] [CrossRef]
- He, M.Y.; Sun, Y.H.; Han, B.X. Green Carbon Science: Scientific Basis for Integrating Carbon Resource Processing, Utilization, and Recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef]
- Yang, Z.Z.; He, L.N.; Gao, J.; Liu, A.H.; Yu, B. Carbon dioxide utilization with C–N bond formation: Carbon dioxide capture and subsequent conversion. Energy Environ. Sci. 2012, 5, 6602–6639. [Google Scholar] [CrossRef]
- Navarrete-Vazquez, G.; Cedillo, R.; Hernandez-Campos, A.; Yepez, L.; Hernandez-Luis, F.; Valdez, J.; Morales, R.; Cortes, R.; Hernandez, M.; Castillo, R. Synthesis and antiparasitic activity of 2-(trifluoromethyl) benzimidazole derivatives. Biorg. Med. Chem. Lett. 2001, 11, 187–190. [Google Scholar] [CrossRef]
- Derayea, S.M.; Ali, H.R.H.; Hamad, A.A.; Ali, R.J. Application of silver nanoparticles for the spectrophotometric determination of three benzimidazole anthelmintic drugs in their pharmaceutical preparations. Appl. Pharm. Sci. 2017, 7, 76–82. [Google Scholar]
- Khatun, R.; Biswas, S.; Biswas, I.H.; Riyajuddin, S.; Haque, N.; Ghosh, K.; Islam, S.M. Cu-NPs@COF: A potential heterogeneous catalyst for CO2 fixation to produce 2-oxazolidinones as well as benzimidazoles under moderate reaction conditions. J. CO2 Util. 2020, 40, 101180–101191. [Google Scholar] [CrossRef]
- Wang, F.; He, S.; Chen, H.; Wang, B.; Zheng, L.; Wei, M.; Evans, D.G.; Duan, X. Active Site Dependent Reaction Mechanism over Ru/CeO2 Catalyst toward CO2 Methanation. J. Am. Chem. Soc. 2016, 138, 6298–6305. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-Based Catalysts for CO2 Methanation: A Review. Nanomaterials 2021, 11, 1–34. [Google Scholar] [CrossRef]
- Guo, Y.; Mei, S.; Yuan, K.; Wang, D.-J.; Liu, H.-C.; Yan, C.-H.; Zhang, Y.-W. Low-Temperature CO2 Methanation over CeO2-Supported Ru. ACS Catal. 2018, 8, 6203–6215. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, H.Y.; Zhao, Y.F.; Chen, S.; Xu, J.L.; Huang, C.L.; Liu, Z.M. Cyclization of o-phenylenediamines by CO2 in the presence of H2 for the synthesis of benzimidazoles. Green Chem. 2013, 15, 95–99. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Widmann, D.; Olesen, S.E.; Chorkendorff, I.; Biskupek, J.; Behm, R.J. Selective CO Methanation on Ru/TiO2 Catalysts. ACS Catal. 2015, 5, 6753–6763. [Google Scholar] [CrossRef]
- Tsang, S.; Claridge, J.B.; Green, M. Recent advances in the conversion of methane to synthesis gas. Catal. Today 1995, 23, 3–15. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef]
- Qin, N.; Pan, A.; Yuan, J.; Ke, F.; Wu, X.; Zhu, J.; Liu, J.; Zhu, J. One-Step Construction of a Hollow Au@Bimetal-Organic Framework Core-Shell Catalytic Nanoreactor for Selective Alcohol Oxidation Reaction. ACS Appl. Mater. Interfaces 2021, 13, 12463–12471. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Ariga, K.; Yamauchi, Y.J. A new family of carbon materials: Synthesis of MOF-derived nanoporous carbons and their promising applications. Mater. Chem. A 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Lin, X.; Wang, S.; Tu, W.; Hu, Z.; Ding, Z.; Hou, Y.; Dai, W. MOF-derived hierarchical hollow spheres composed of carbon-confined Ni nanoparticles for efficient CO2 methanation. Catal. Sci. Technol. 2019, 9, 731–738. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Pan, Q.; Yu, J.; Xu, R. Three metal-organic frameworks prepared from mixed solvents of DMF and HAc. Microporous Mesoporous Mater. 2006, 90, 145–152. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, G. Metal–Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Hamza Fakeeha, A.; Arafat, Y.; Aidid Ibrahim, A.; Shaikh, H.; Atia, H.; Elhag Abasaeed, A.; Armbruster, U.; Sadeq Al-Fatesh, A. Highly Selective Syngas/H2 Production via Partial Oxidation of CH4 Using (Ni, Co and Ni–Co)/ZrO2–Al2O3 Catalysts: Influence of Calcination Temperature. Processes 2019, 7, 141. [Google Scholar] [CrossRef]
- Long, J.; Shen, K.; Chen, L.; Li, Y.J. Multimetal-MOF-derived transition metal alloy NPs embedded in an N-doped carbon matrix: Highly active catalysts for hydrogenation reactions. Mater. Chem. A 2016, 4, 10254–10262. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H.; Groy, T.L. Construction of Porous Solids from Hydrogen-Bonded Metal Complexes of 1,3,5-Benzenetricarboxylic acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- Zhao, W.; Li, H.; Li, Y.; Long, J.; Xu, Y.; Yang, S. Low-cost acetate-catalyzed efficient synthesis of benzimidazoles using ambient CO2 as a carbon source under mild conditions. Sustain. Chem. Pharm. 2020, 17, 100276. [Google Scholar] [CrossRef]
- Hao, L.; Zhao, Y.; Yu, B.; Zhang, H.; Xu, H.; Liu, Z. Au catalyzed synthesis of benzimidazoles from 2-nitroanilines and CO2/H2. Green Chem. 2014, 16, 3039. [Google Scholar] [CrossRef]
- Biswas, I.H.; Biswas, S.; Islam, M.S.; Riyajuddin, S.; Sarkar, P.; Ghosh, K.; Islam, S.M. Catalytic synthesis of benzimidazoles and organic carbamates using a polymer supported zinc catalyst through CO2 fixation. New J. Chem. 2019, 43, 14643. [Google Scholar] [CrossRef]
- Ke, Z.; Yu, Z.; Wang, H.; Xiang, J.; Han, J.; Wu, Y.; Liu, Z.; Yang, P.; Liu, Z. Cobalt-catalyzed synthesis of N-containing heterocycles via cyclization of ortho-substituted anilines with CO2/H2. Green Chem. 2019, 21, 1695. [Google Scholar] [CrossRef]
- Phatake, V.V.; Bhanage, B.M. Cu@UgC3N4 Catalyzed Cyclization of o-Phenylenediamines for the Synthesis of Benzimidazoles by Using CO2 and Dimethylamine Borane as a Hydrogen. Catal. Lett. 2019, 149, 347. [Google Scholar] [CrossRef]
- Rasal, K.B.; Yadav, G.D. One-pot synthesis of benzimidazole using DMF as a multitasking reagent in presence CuFe2O4 as catalyst. Catal. Today 2018, 309, 51. [Google Scholar] [CrossRef]
- Mihet, M.; Dan, M.; Barbu-Tudoran, L.; Lazar, M.D. CO2 Methanation Using Multimodal Ni/SiO2 Catalysts: Effect of Support Modification by MgO, CeO2, and La2O3. Catalysts 2021, 11, 443. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Xing, Y.; Xu, S.; Xie, H.; Xiong, K. CO2 hydrogenation to methane over mesoporous Co/SiO2 catalysts: Effect of structure. J. CO2 Util. 2018, 26, 221. [Google Scholar] [CrossRef]
- Liu, H.; Xu, S.; Zhou, G.; Xiong, K.; Jiao, Z.; Wang, S. CO2 hydrogenation to methane over Co/KIT-6 catalysts: Effect of Co content. Fuel 2018, 217, 570–576. [Google Scholar] [CrossRef]
- Pieta, I.S.; Lewalska-Graczyk, A.; Kowalik, P.; Antoniak-Jurak, K. CO2 Hydrogenation to Methane over Ni-Catalysts: The Effect of Support and Vanadia Promoting. Catalysts. 2021, 11, 433. [Google Scholar] [CrossRef]
- Varvoutis, G.; Lykaki, M.; Stefa, S.; Papista, E.; Carabineiro, S.A.; Marnellos, G.E.; Konsolakis, M. Remarkable efficiency of Ni supported on hydrothermally synthesized CeO2 nanorods for low-temperature CO2 hydrogenation to methane. Catal. Commun. 2020, 142, 106036. [Google Scholar] [CrossRef]
- Franken, T.; Terreni, J.; Borgschulte, A.; Heel, A. Solid solutions in reductive environment–A case study on improved CO2 hydrogenation to methane on cobalt based catalysts derived from ternary mixed metal oxides. J. Catal. 2020, 382, 385. [Google Scholar] [CrossRef]
- Dong, T.; Liu, X.; Tang, Z.; Yuan, H.; Jiang, D.; Wang, Y.; Liu, Z.; Zhang, X.; Huang, S.; Liu, H.; et al. Ru Decorated TiOx Nanoparticles via Laser Bombardment for Photothermal Co-catalytic CO2 Hydrogenation to Methane with High Selectivity. Appl. Catal B Environ. 2022, 326, 122176. [Google Scholar] [CrossRef]









 | |||
|---|---|---|---|
| Entry | Catalysts | Temperature/°C | Yield/% b |
| 1 | ---- | 115 | 0 |
| 2 | Ni(NO3)26H2O | 115 | 0 |
| 3 | Co(NO3)26H2O | 115 | 0 |
| 4 | CoNiBTC | 115 | 9 |
| 5 | CoNiBTC | 130 | 11 |
| 6 | Co@C | 115 | 40 |
| 7 | Ni@C | 115 | 33 |
| 8 | CoNi@C | 115 | 81 |
| 9 | CoNi@C-1 | 115 | 52 |
| 10 | CoNi@C-2 | 115 | 38 |
| Entry | Substrate | Product | Yield |
|---|---|---|---|
| 1 |  |  | 81 |
| 2 |  |  | 78 |
| 3 |  |  | 82 |
| 4 |  |  | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helal, A.; Sanhoob, M.A.; Hoque, B.; Usman, M.; Zahir, M.H. Bimetallic Metal-Organic Framework Derived Nanocatalyst for CO2 Fixation through Benzimidazole Formation and Methanation of CO2. Catalysts 2023, 13, 357. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13020357
Helal A, Sanhoob MA, Hoque B, Usman M, Zahir MH. Bimetallic Metal-Organic Framework Derived Nanocatalyst for CO2 Fixation through Benzimidazole Formation and Methanation of CO2. Catalysts. 2023; 13(2):357. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13020357
Chicago/Turabian StyleHelal, Aasif, Mohammed Ahmed Sanhoob, Bosirul Hoque, Muhammad Usman, and Md. Hasan Zahir. 2023. "Bimetallic Metal-Organic Framework Derived Nanocatalyst for CO2 Fixation through Benzimidazole Formation and Methanation of CO2" Catalysts 13, no. 2: 357. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13020357