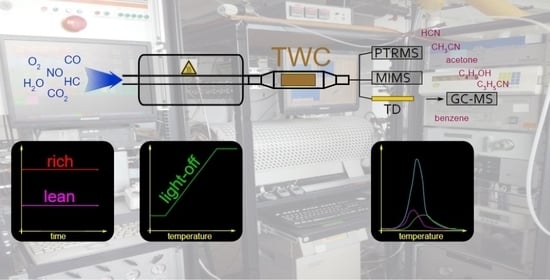
Analysis of Unregulated VOCs Downstream a Three-Way Catalyst in a Simulated Gasoline Engine Exhaust under Non-Optimum Conditions
Abstract
:1. Introduction
2. Results
2.1. Catalytic Performances
2.2. Nitrous Oxide Formation
2.3. VOCs Formation at Constant Temperature
2.4. VOCs Emissions Analysis during a Light-Off Experiment
3. Materials and Methods
3.1. Synthetic Gas Bench and Reactor
3.2. Analyzers
3.3. Mass Spectrometry Analysis
3.4. Light-Off Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TWC | Three-Way Catalyst |
| VOC | Volatile Organic Compound |
| SGB | Synthetic Gas Bench |
| MIMS | Membrane Inlet Mass Spectrometry |
| PTR-MS | Proton Transfer Reaction Mass Spectrometry |
| TD-GC-MS | ThermoDersorption-Gas Chromatrography-Mass Spectrometry |
| LO | Light-off |
References
- Suarez-Bertoa, R.; Lähde, T.; Pavlovic, J.; Valverde, V.; Clairotte, M.; Giechaskiel, B. Laboratory and On-Road Evaluation of a GPF-Equipped Gasoline Vehicle. Catalysts 2019, 9, 678. [Google Scholar] [CrossRef] [Green Version]
- Martinet, S.; Liu, Y.; Louis, C.; Tassel, P.; Perret, P.; Chaumond, A.; André, M. Euro 6 Unregulated Pollutant Characterization and Statistical Analysis of After-Treatment Device and Driving-Condition Impact on Recent Passenger-Car Emissions. Environ. Sci. Technol. 2017, 51, 5847–5855. [Google Scholar] [CrossRef] [Green Version]
- Clairotte, M.; Adam, T.W.; Chirico, R.; Giechaskiel, B.; Manfredi, U.; Elsasser, M.; Sklorz, M.; DeCarlo, P.F.; Heringa, M.F.; Zimmermann, R.; et al. Online characterization of regulated and unregulated gaseous and particulate exhaust emissions from two-stroke mopeds: A chemometric approach. Anal. Chim. Acta 2012, 717, 28–38. [Google Scholar] [CrossRef]
- Shelef, M.; McCabe, R.W. Twenty-five years after introduction of automotive catalysts: What next? Catal. Today 2000, 62, 35–50. [Google Scholar] [CrossRef]
- Rood, S.; Eslava, S.; Manigrasso, A.; Bannister, C. Recent advances in gasoline three-way catalyst formulation: A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2020, 234, 936–949. [Google Scholar] [CrossRef]
- Reiter, M.S.; Kockelman, K.M. The problem of cold starts: A closer look at mobile source emissions levels. Transp. Res. Part D Transp. Environ. 2016, 43, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Glorennec, P.; Bonvallot, N.; Mandin, C.; Goupil, G.; Pernelet-Joly, V.; Millet, M.; Filleul, L.; Le Moullec, Y.; Alary, R. Is a quantitative risk assessment of air quality in underground parking garages possible? Indoor Air 2008, 18, 283–292. [Google Scholar] [CrossRef]
- Lee, J.; Theis, J.R.; Kyriakidou, E.A. Vehicle emissions trapping materials: Successes, challenges, and the path forward. Appl. Catal. B Environ. 2019, 243, 397–414. [Google Scholar] [CrossRef]
- Getsoian, A.B.; Theis, J.R.; Lambert, C.K. Sensitivity of Three-Way Catalyst Light-Off Temperature to Air-Fuel Ratio. Emiss. Control Sci. Technol. 2018, 4, 136–142. [Google Scholar] [CrossRef]
- Baum, M.M.; Moss, J.A.; Pastel, S.H.; Poskrebyshev, G.A. Hydrogen Cyanide Exhaust Emissions from In-Use Motor Vehicles. Environ. Sci. Technol. 2007, 41, 857–862. [Google Scholar] [CrossRef]
- Poulopoulos, S.G.; Samaras, D.P.; Philippopoulos, C.J. Regulated and speciated hydrocarbon emissions from a catalyst equipped internal combustion engine. Atmos. Environ. 2001, 35, 4443–4450. [Google Scholar] [CrossRef]
- Hasan, A.O.; Abu-jrai, A.; Al-Muhtaseb, A.H.; Tsolakis, A.; Xu, H. Formaldehyde, acetaldehyde and other aldehyde emissions from HCCI/SI gasoline engine equipped with prototype catalyst. Fuel 2016, 175, 249–256. [Google Scholar] [CrossRef]
- Schmitz, T.; Hassel, D.; Weber, F.J. Determination of VOC-components in the exhaust of gasoline and diesel passenger cars. Atmos. Environ. 2000, 34, 4639–4647. [Google Scholar] [CrossRef]
- Hata, H.; Okada, M.; Funakubo, C.; Hoshi, J. Tailpipe VOC Emissions from Late Model Gasoline Passenger Vehicles in the Japanese Market. Atmosphere 2019, 10, 621. [Google Scholar] [CrossRef] [Green Version]
- Nandi, S.; Arango, P.; Chaillou, C.; Dujardin, C.; Granger, P.; Laigle, E.; Nicolle, A.; Norsic, C.; Richard, M. Relationship between design strategies of commercial three-way monolithic catalysts and their performances in realistic conditions. Catal. Today 2022, 384–386, 122–132. [Google Scholar] [CrossRef]
- Keirns, M.H.; Holt, E.L. Hydrogen Cyanide Emissions from Three-Way Catalyst Prototypes under Malfunctioning Conditions. SAE Trans. 1978, 87, 815–827. [Google Scholar] [CrossRef]
- Jang, J.; Lee, J.; Choi, Y.; Park, S. Reduction of particle emissions from gasoline vehicles with direct fuel injection systems using a gasoline particulate filter. Sci. Total Environ. 2018, 644, 1418–1428. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, D.; Tsampas, M.N.; Boréave, A.; Retailleau-Mevel, L.; Klotz, M.; Tardivat, C.; Cartoixa, B.; Pajot, K.; Vernoux, P. Mixed Ionic–Electronic Conducting Catalysts for Catalysed Gasoline Particulate Filters. Top. Catal. 2015, 58, 1242–1255. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A.; Kanter, D. Inventories and scenarios of nitrous oxide emissions. Environ. Res. Lett. 2014, 9, 105012. [Google Scholar] [CrossRef]
- Dasch, J.M. Nitrous Oxide Emissions from Vehicles. J. Air Waste Manag. Assoc. 1992, 42, 63–67. [Google Scholar] [CrossRef]
- Srinivasan, A.; Depcik, C. Review of Chemical Reactions in the NO Reduction by CO on Rhodium/Alumina Catalysts. Catal. Rev. 2010, 52, 462–493. [Google Scholar] [CrossRef]
- Buhr, K.; van Ruth, S.; Delahunty, C. Analysis of volatile flavour compounds by Proton Transfer Reaction-Mass Spectrometry: Fragmentation patterns and discrimination between isobaric and isomeric compounds. Int. J. Mass Spectrom. 2002, 221, 1–7. [Google Scholar] [CrossRef]
- Brazdil, J.F. A critical perspective on the design and development of metal oxide catalysts for selective propylene ammoxidation and oxidation. Appl. Catal. A Gen. 2017, 543, 225–233. [Google Scholar] [CrossRef]
- Giordano, N.; Bart, J. On the mechanism of ammoxidation of propene over cerium-doped bismuth molybdate catalysts. Recl. Des Trav. Chim. Des Pays-Bas 1975, 94, 28–30. [Google Scholar] [CrossRef]
- Karlsson, H.L. Ammonia, nitrous oxide and hydrogen cyanide emissions from five passenger vehicles. Sci. Total. Environ. 2004, 334–335, 125–132. [Google Scholar] [CrossRef]
- Moussa, S.G.; Leithead, A.; Li, S.M.; Chan, T.W.; Wentzell, J.J.B.; Stroud, C.; Zhang, J.; Lee, P.; Lu, G.; Brook, J.R.; et al. Emissions of hydrogen cyanide from on-road gasoline and diesel vehicles. Atmos. Environ. 2016, 131, 185–195. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Staebler, R.M.; Moussa, S.G.; Su, Y.; Munoz, T.; Stroud, C.; Zhang, J.; Moran, M.D. Long-path measurements of pollutants and micrometeorology over Highway 401 in Toronto. Atmos. Chem. Phys. 2017, 17, 14119–14143. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.R.; Jennings, T.J. Investigation of the mechanism of catalytic oxidation of propylene to acrolein and acrylonitrile. J. Catal. 1963, 2, 63–68. [Google Scholar] [CrossRef]
- Zervas, E.; Montagne, X.; Lahaye, J. Emission of Alcohols and Carbonyl Compounds from a Spark Ignition Engine. Influence of Fuel and Air/Fuel Equivalence Ratio. Environ. Sci. Technol. 2002, 36, 2414–2421. [Google Scholar] [CrossRef]
- Lizarraga, L.; Souentie, S.; Boreave, A.; George, C.; D’Anna, B.; Vernoux, P. Effect of Diesel Oxidation Catalysts on the Diesel Particulate Filter Regeneration Process. Environ. Sci. Technol. 2011, 45, 10591–10597. [Google Scholar] [CrossRef]
- Davey, N.G.; Krogh, E.T.; Gill, C.G. Membrane-introduction mass spectrometry (MIMS). TrAC Trends Anal. Chem. 2011, 30, 1477–1485. [Google Scholar] [CrossRef]
- Louarn, E.; Hamrouni, A.; Colbeau-Justin, C.; Bruschi, L.; Lemaire, J.; Heninger, M.; Mestdagh, H. Characterization of a membrane inlet interfaced with a compact chemical ionization FT-ICR for real-time and quantitative VOC analysis in water. Int. J. Mass Spectrom. 2013, 353, 26–35. [Google Scholar] [CrossRef]






| CO | NO | C3H6 | C3H8 | O2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R00 | 231 | 260 | 243 | 299 | 257 | 351 | 462 | 535 | 228 | 260 |
| R30 | 231 | 254 | 242 | 286 | 252 | 335 | 433 | 566 | 230 | 255 |
| R37 | 217 | 246 | 235 | 282 | 246 | 330 | 396 | 457 | 214 | 247 |
| L37 | 215 | 250 | 257 | - | 245 | 257 | 299 | 402 | 242 | 260 |
| Rich | Lean | |
|---|---|---|
| O2 | 0.4% | 1% |
| CO | 6000 ppm | 4000 ppm |
| NO | 600 ppm | 600 ppm |
| C3H6 | 1125 ppm | 1125 ppm |
| C3H8 | 375 ppm | 375 ppm |
| H2O | X = 0% (dry) or 3% (wet) | |
| CO2 | Y = 0% or 7% | |
| Total flux | 20 NL min−1 in N2 | |
| MS Tech. | Sampling | Dilution | Gas Conditions | Temperature () |
|---|---|---|---|---|
| MIMS | Direct sampling through a semi-permeable membrane; response time <1 min | no | Rich and lean according to Table 2, in dry and wet conditions | Ramp |
| PTR-MS | Direct sampling; response time under seconds for one spectrum, but needs 20 min accumulation time in order to decrease detection limit | Rich but with modified values of HC (C3H6: 312 ppm and C3H8: 104 ppm) and O2 | Stepwise increase of temperature: 150 °C, 280 °C, 400 °C, 500 °C, 670 °C | |
| TD-GC-MS | Adsorption on filled tube (Tenax or Carbotrap-carbosieve); adsorption time was 1 h | no | Rich according to Table 2, in dry and wet conditions | At 300 °C and 400 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louarn, E.; Boreave, A.; Raffin, G.; George, C.; Vernoux, P. Analysis of Unregulated VOCs Downstream a Three-Way Catalyst in a Simulated Gasoline Engine Exhaust under Non-Optimum Conditions. Catalysts 2023, 13, 563. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13030563
Louarn E, Boreave A, Raffin G, George C, Vernoux P. Analysis of Unregulated VOCs Downstream a Three-Way Catalyst in a Simulated Gasoline Engine Exhaust under Non-Optimum Conditions. Catalysts. 2023; 13(3):563. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13030563
Chicago/Turabian StyleLouarn, Essyllt, Antoinette Boreave, Guy Raffin, Christian George, and Philippe Vernoux. 2023. "Analysis of Unregulated VOCs Downstream a Three-Way Catalyst in a Simulated Gasoline Engine Exhaust under Non-Optimum Conditions" Catalysts 13, no. 3: 563. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13030563