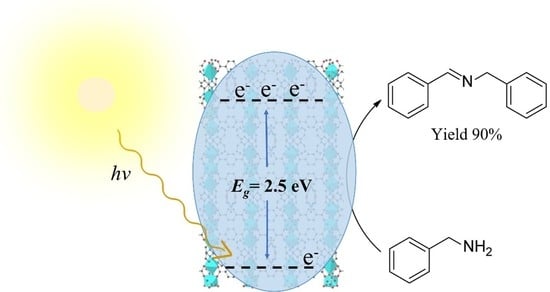
Manganese-Based Metal-Organic Frameworks Photocatalysts for Visible Light-Driven Oxidative Coupling of Benzylamine under Atmospheric Oxygen: A Comparative Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Strucutres Details
2.2. Powder X-ray Diffraction (PXRD)
2.3. Scanning Electron Microscope (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX)
2.4. UV−Vis Diffuse Reflectance Spectroscopy (UV−Vis DRS)
2.5. Thermal Gravimetric Analysis (TGA)
2.6. Stability Test
2.7. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.8. MnII3(tp)6/2(bpy)2.(dmf) Photocatalytic Activity
3. Experimental Section
3.1. Materials and General Procedures
3.2. Synthesis of MnII3(tp)6/2(bpy)2.(dmf)
3.3. Synthesis of Mn2(tpa)2(dmf)2 MOF
3.4. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor Heterojunction Photocatalysts: Design, Construction, and Photocatalytic Performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Ma, L.; Yang, X.; Cao, J. Recent Advances in Visible-Light Photocatalytic Deuteration Reactions. Org. Chem. Front. 2021, 8, 426–444. [Google Scholar] [CrossRef]
- Chen, H.; Wan, K.; Zheng, F.; Zhang, Z.; Zhang, Y.; Long, D. Mechanism Insight into Photocatalytic Conversion of Lignin for Valuable Chemicals and Fuels Production: A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2021, 147, 111217. [Google Scholar] [CrossRef]
- Stroyuk, O.L.; Kuchmy, S.Y. Heterogeneous Photocatalytic Selective Reductive Transformations of Organic Compounds: A Review. Theor. Exp. Chem. 2020, 56, 143–173. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.E.K.; Bouhfid, R. Recent Progress on Ag/TiO2 Photocatalysts: Photocatalytic and Bactericidal Behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef]
- Bakiro, M.; Ahmed, S.H.; Alzamly, A. Cycloaddition of CO2 to Propylene Oxide Using BiNbO4/NH2-MIL-125(Ti) Composites as Visible-Light Photocatalysts. J. Environ. Chem. Eng. 2020, 8, 104461. [Google Scholar] [CrossRef]
- Bakiro, M.; Ahmed, S.H.; Alzamly, A. Investigation of the Band Gap Energy Shift and Photocatalytic Properties of Bi3+-Doped Ceria. Inorg. Chem. Commun. 2020, 116, 107906. [Google Scholar] [CrossRef]
- Alzamly, A.; Bakiro, M.; Ahmed, S.H.; Sallabi, S.M.; Al Ajeil, R.A.; Alawadhi, S.A.; Selem, H.A.; Al Meshayei, S.S.M.; Khaleel, A.; Al-Shamsi, N.; et al. Construction of BiOF/BiOI Nanocomposites with Tunable Band Gaps as Efficient Visible-Light Photocatalysts. J. Photochem. Photobiol. A Chem. 2019, 375, 30–39. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, T.; Wang, J.; Wang, Y.; Chen, Z.; Liu, B.; Ji, H.; Wang, W.; Zhang, G.; Li, Y. Fabrication of G-C3N4/PW12/TiO2 Composite with Significantly Enhanced Photocatalytic Performance under Visible Light. J. Alloys Compd. 2021, 860, 157924. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent Advances in MOF-Based Photocatalysis: Environmental Remediation under Visible Light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal Oxides as Photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.M.; Rahman, A.; Matussin, S.N. Recent Progress of Metal-Organic Frameworks and Metal-Organic Frameworks-Based Heterostructures as Photocatalysts. Nanomaterials 2022, 12, 2820. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular Synthesis and the Design of New Materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal–Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhou, A.; Dou, Y.; Zhou, J.; Bai, J.; Li, J.-R. Dual MOFs Template-Directed Fabrication of Hollow-Structured Heterojunction Photocatalysts for Efficient CO2 Reduction. Chem. Eng. J. 2021, 416, 129155. [Google Scholar] [CrossRef]
- Abdul Mubarak, N.S.; Foo, K.Y.; Schneider, R.; Abdelhameed, R.M.; Sabar, S. The Chemistry of MIL-125 Based Materials: Structure, Synthesis, Modification Strategies and Photocatalytic Applications. J. Environ. Chem. Eng. 2022, 10, 106883. [Google Scholar] [CrossRef]
- Du, P.D.; Thanh, H.T.M.; To, T.C.; Thang, H.S.; Tinh, M.X.; Tuyen, T.N.; Hoa, T.T.; Khieu, D.Q. Metal-Organic Framework MIL-101: Synthesis and Photocatalytic Degradation of Remazol Black B Dye. J. Nanomater. 2019, 2019, 6061275. [Google Scholar] [CrossRef] [Green Version]
- Siddig, L.A.; Alzard, R.H.; Nguyen, H.L.; Göb, C.R.; Alnaqbi, M.A.; Alzamly, A. Hexagonal Layer Manganese Metal–Organic Framework for Photocatalytic CO2 Cycloaddition Reaction. ACS Omega 2022, 7, 9958–9963. [Google Scholar] [CrossRef]
- Laurier, K.G.M.; Vermoortele, F.; Ameloot, R.; De Vos, D.E.; Hofkens, J.; Roeffaers, M.B.J. Iron(III)-Based Metal-Organic Frameworks as Visible Light Photocatalysts. J. Am. Chem. Soc. 2013, 135, 14488–14491. [Google Scholar] [CrossRef]
- Nguyen, H.L. Reticular Materials for Artificial Photoreduction of CO2. Adv. Energy Mater. 2020, 10, 1–23. [Google Scholar] [CrossRef]
- Shen, Q.; Li, X.; Li, R.; Wu, Y. Application of Metal–Organic Framework Materials and Derived Porous Carbon Materials in Catalytic Hydrogenation. ACS Sustain. Chem. Eng. 2020, 8, 17608–17621. [Google Scholar] [CrossRef]
- Alzard, R.H.; Siddig, L.A.; Alhatti, N.; Abdallah, I.; Aljabri, L.; Alblooshi, A.; Alzamly, A. Titania Derived from NH2-MIL-125(Ti) Metal–Organic Framework for Selective Photocatalytic Conversion of CO2 to Propylene Carbonate. Comments Inorg. Chem. 2022, 43, 1–15. [Google Scholar] [CrossRef]
- Alzard, R.H.; Siddig, L.A.; Abdelhamid, A.S.; Alzamly, A. Visible-Light-Driven Photocatalytic Coupling of Neat Benzylamine over a Bi-Ellagate Metal–Organic Framework. ACS Omega 2022, 7, 36689–36696. [Google Scholar] [CrossRef]
- Li, M.; Yuan, J.; Wang, G.; Yang, L.; Shao, J.; Li, H.; Lu, J. One-Step Construction of Ti-In Bimetallic MOFs to Improve Synergistic Effect of Adsorption and Photocatalytic Degradation of Bisphenol A. Sep. Purif. Technol. 2022, 298, 121658. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, X.; Ma, S.; Xia, X.; Shi, Y.; Yang, J. Preparation and Application of Bimetallic Mixed Ligand MOF Photocatalytic Materials. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 636, 128108. [Google Scholar] [CrossRef]
- Somnath; Ahmad, M.; Siddiqui, K.A. Synthesis of Mixed Ligand 3D Cobalt MOF: Smart Responsiveness towards Photocatalytic Dye Degradation in Environmental Contaminants. J. Mol. Struct. 2022, 1265, 133399. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Guo, W.; Wang, Z.; Shi, Y.; Yu, Y.; Wu, L. Functionalized UiO-66(Ce) for Photocatalytic Organic Transformation: The Role of Active Sites Modulated by Ligand Functionalization. Catal. Sci. Technol. 2022, 12, 1812–1823. [Google Scholar] [CrossRef]
- Han, W.; Shao, L.-H.; Sun, X.-J.; Liu, Y.-H.; Zhang, F.-M.; Wang, Y.; Dong, P.-Y.; Zhang, G.-L. Constructing Cu Ion Sites in MOF/COF Heterostructure for Noble-Metal-Free Photoredox Catalysis. Appl. Catal. B Environ. 2022, 317, 121710. [Google Scholar] [CrossRef]
- Ye, Z.; Feng, S.; Wu, W.; Zhou, Y.; Wang, Y.; Dai, X.; Cao, X. Synthesis of Double MOFs Composite Material for Visible Light Photocatalytic Degradation of Tetracycline. Solid State Sci. 2022, 127, 106842. [Google Scholar] [CrossRef]
- Lv, S.-W.; Liu, J.-M.; Li, C.-Y.; Zhao, N.; Wang, Z.-H.; Wang, S. Two Novel MOFs@COFs Hybrid-Based Photocatalytic Platforms Coupling with Sulfate Radical-Involved Advanced Oxidation Processes for Enhanced Degradation of Bisphenol A. Chemosphere 2020, 243, 125378. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Li, Y.; Xu, X.; Du, Y.; Jiang, Y.; Lin, K. A Novel Heterostructure Coupling MOF-Derived Fluffy Porous Indium Oxide with g-C3N4 for Enhanced Photocatalytic Activity. Mater. Res. Bull. 2021, 133, 111078. [Google Scholar] [CrossRef]
- Lu, W.; Duan, C.; Liu, C.; Zhang, Y.; Meng, X.; Dai, L.; Wang, W.; Yu, H.; Ni, Y. A Self-Cleaning and Photocatalytic Cellulose-Fiber-Supported “Ag@AgCl@MOF-Cloth’’ Membrane for Complex Wastewater Remediation. Carbohydr. Polym. 2020, 247, 116691. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud Idris, A.; Jiang, X.; Tan, J.; Cai, Z.; Lou, X.; Wang, J.; Li, Z. Dye-Sensitized Fe-MOF Nanosheets as Visible-Light Driven Photocatalyst for High Efficient Photocatalytic CO2 Reduction. J. Colloid Interface Sci. 2022, 607, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Cai, X.; Jiang, H.L. Improving MOF Stability: Approaches and Applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.; Qin, L.; You, L.; Yao, Y.; Wu, H.; Zhang, Z.; Zhang, L.; Yin, X.; Ni, J. Functionalization of MOF-5 with Mono-Substituents: Effects on Drug Delivery Behavior. RSC Adv. 2020, 10, 36862–36872. [Google Scholar] [CrossRef]
- Bahamon, D.; Anlu, W.; Builes, S.; Khaleel, M.; Vega, L.F. Effect of Amine Functionalization of MOF Adsorbents for Enhanced CO2 Capture and Separation: A Molecular Simulation Study. Front. Chem. 2021, 8, 574–622. [Google Scholar] [CrossRef]
- Sunder, N.; Fong, Y.Y.; Bustam, M.A.; Suhaimi, N.H. Development of Amine-Functionalized Metal-Organic Frameworks Hollow Fiber Mixed Matrix Membranes for CO2 and CH4 Separation: A Review. Polymers 2022, 14, 1408. [Google Scholar] [CrossRef]
- Mulfort, K.L.; Farha, O.K.; Malliakas, C.D.; Kanatzidis, M.G.; Hupp, J.T. An Interpenetrated Framework Material with Hysteretic CO2 Uptake. Chem. A Eur. J. 2010, 16, 276–281. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, M.; Li, D. A Dynamic, Luminescent and Entangled MOF as a Qualitative Sensor for Volatile Organic Solvents and a Quantitative Monitor for Acetonitrile Vapour. Chem. Sci. 2013, 4, 1793–1801. [Google Scholar] [CrossRef]
- Haldar, R.; Maji, T.K. Metal-Organic Frameworks (MOFs) Based on Mixed Linker Systems: Structural Diversities towards Functional Materials. CrystEngComm 2013, 15, 9276–9295. [Google Scholar] [CrossRef]
- Bunck, D.N.; Dichtel, W.R. Mixed Linker Strategies for Organic Framework Functionalization. Chem. A Eur. J. 2013, 19, 818–827. [Google Scholar] [CrossRef]
- Qin, J.S.; Yuan, S.; Wang, Q.; Alsalme, A.; Zhou, H.C. Mixed-Linker Strategy for the Construction of Multifunctional Metal-Organic Frameworks. J. Mater. Chem. A 2017, 5, 4280–4291. [Google Scholar] [CrossRef]
- Chun, H.; Dybtsev, D.N.; Kim, H.; Kim, K. Synthesis, X-ray Crystal Structures, and Gas Sorption Properties of Pillared Square Grid Nets Based on Paddle-Wheel Motifs: Implications for Hydrogen Storage in Porous Materials. Chem. A Eur. J. 2005, 11, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Chelucci, G.; Thummel, R.P. Chiral 2,2‘-Bipyridines, 1,10-Phenanthrolines, and 2,2‘:6‘,2‘‘-Terpyridines: Syntheses and Applications in Asymmetric Homogeneous Catalysis. Chem. Rev. 2002, 102, 3129–3170. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.N.; Nguyen, M.V.; Nguyen, H.L.; Yuliarto, B.; Cordova, K.E.; Demir, S. Designing Bipyridine-Functionalized Zirconium Metal–Organic Frameworks as a Platform for Clean Energy and Other Emerging Applications. Coord. Chem. Rev. 2018, 364, 33–50. [Google Scholar] [CrossRef]
- Manna, K.; Zhang, T.; Greene, F.X.; Lin, W. Bipyridine- and Phenanthroline-Based Metal-Organic Frameworks for Highly Efficient and Tandem Catalytic Organic Transformations via Directed C-H Activation. J. Am. Chem. Soc. 2015, 137, 2665–2673. [Google Scholar] [CrossRef]
- Manna, K.; Zhang, T.; Lin, W. Postsynthetic Metalation of Bipyridyl-Containing Metal-Organic Frameworks for Highly Efficient Catalytic Organic Transformations. J. Am. Chem. Soc. 2014, 136, 6566–6569. [Google Scholar] [CrossRef]
- Kent, C.A.; Mehl, B.P.; Ma, L.; Papanikolas, J.M.; Meyer, T.J.; Lin, W. Energy Transfer Dynamics in Metal−Organic Frameworks. J. Am. Chem. Soc. 2010, 132, 12767–12769. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Z.; deKrafft, K.E.; Lin, W. Doping Metal–Organic Frameworks for Water Oxidation, Carbon Dioxide Reduction, and Organic Photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef]
- Maza, W.A.; Ahrenholtz, S.R.; Epley, C.C.; Day, C.S.; Morris, A.J. Solvothermal Growth and Photophysical Characterization of a Ruthenium(II) Tris(2,2′-Bipyridine)-Doped Zirconium UiO-67 Metal Organic Framework Thin Film. J. Phys. Chem. C 2014, 118, 14200–14210. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, X.; Lu, Y.; Yang, Y.; Zhang, Y.P.; Tang, H.L.; Zhang, F.M.; Yang, Z.D.; Sun, X.; Feng, Y. Regulation of Metal Ions in Smart Metal-Cluster Nodes of Metal-Organic Frameworks with Open Metal Sites for Improved Photocatalytic CO2 Reduction Reaction. Appl. Catal. B Environ. 2020, 276, 119173. [Google Scholar] [CrossRef]
- Thulasi Karunakaran, S.; Pavithran, R.; Sajeev, M.; Mohan Mohan Rema, S. Photocatalytic Degradation of Methylene Blue Using a Manganese Based Metal Organic Framework. Results Chem. 2022, 4, 100504. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Y.; Huang, T.; Su, T.; Fu, D.; Yue, S.; Zeng, H. Application of an Mn-MOF as a Highly Efficient Catalyst for Sunlight-Driven Hydrogen Generation. Phase Transitions 2018, 91, 1179–1187. [Google Scholar] [CrossRef]
- Lu, X.M.; Li, P.Z.; Wang, X.T.; Gao, S.; Wang, X.J.; Wang, S.; Deng, Y.H.; Zhang, Y.J.; Zhou, L. Syntheses, Crystal Structures and Magnetic Behaviors of Three MnII-Terephthalate Coordination Polymers Containing Terminal Ligands. Polyhedron 2008, 27, 2402–2408. [Google Scholar] [CrossRef]
- Ladrak, T.; Smulders, S.; Roubeau, O.; Teat, S.J.; Gamez, P.; Reedijk, J. Manganese-Based Metal-Organic Frameworks as Heterogeneous Catalysts for the Cyanosilylation of Acetaldehyde. Eur. J. Inorg. Chem. 2010, 24, 3804–3812. [Google Scholar] [CrossRef]
- Chen, C.X.; Wei, Z.W.; Fan, Y.N.; Su, P.Y.; Ai, Y.Y.; Qiu, Q.F.; Wu, K.; Yin, S.Y.; Pan, M.; Su, C.Y. Visualization of Anisotropic and Stepwise Piezofluorochromism in an MOF Single Crystal. Chem 2018, 4, 2658–2669. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Cao, L.; Hu, X.; Ren, Z.; Zhang, C.; Wang, C. Simulating Powder X-ray Diffraction Patterns of Two-Dimensional Materials. Inorg. Chem. 2018, 57, 15123–15132. [Google Scholar] [CrossRef]
- Leszczyński, M.K.; Justyniak, I.; Gontarczyk, K.; Lewiński, J. Solvent Templating and Structural Dynamics of Fluorinated 2D Cu-Carboxylate MOFs Derived from the Diffusion-Controlled Process. Inorg. Chem. 2020, 59, 4389–4396. [Google Scholar] [CrossRef]
- Guillerm, V.; Weseliński, Ł.J.; Belmabkhout, Y.; Cairns, A.J.; D’Elia, V.; Wojtas, Ł.; Adil, K.; Eddaoudi, M. Discovery and Introduction of a (3,18)-Connected Net as an Ideal Blueprint for the Design of Metal-Organic Frameworks. Nat. Chem. 2014, 6, 673–680. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Presti, D.; Luz, I.; Llabrés i Xamena, F.X.; Bordiga, S. Visible-Light-Driven Photocatalytic Coupling of Benzylamine over Titanium-Based MIL-125-NH2 Metal–Organic Framework: A Mechanistic Study. J. Phys. Chem. C 2020, 124, 23707–23715. [Google Scholar] [CrossRef]
- Sun, D.; Ye, L.; Li, Z. Visible-Light-Assisted Aerobic Photocatalytic Oxidation of Amines to Imines over NH2-MIL-125(Ti). Appl. Catal. B Environ. 2015, 164, 428. [Google Scholar] [CrossRef]
- Yang, X.; Huang, T.; Gao, S.; Cao, R. Boosting Photocatalytic Oxidative Coupling of Amines by a Ru-Complex-Sensitized Metal-Organic Framework. J. Catal. 2019, 378, 248–255. [Google Scholar] [CrossRef]
- Li, J.; Chang, B.; Zhao, H.; Meng, Q.; Li, M.; Han, Q. Visible-Light-Responsive Polyoxometalate-Based Metal–Organic Framework for Highly Efficient Photocatalytic Oxidative Coupling of Amines. J. Mater. Sci. 2021, 56, 6676–6688. [Google Scholar] [CrossRef]
- Su, F.; Mathew, S.C.; Möhlmann, L.; Antonietti, M.; Wang, X.; Blechert, S. Aerobic Oxidative Coupling of Amines by Carbon Nitride Photocatalysis with Visible Light. Angew. Chem. Int. Ed. 2011, 50, 657–660. [Google Scholar] [CrossRef] [PubMed]








 | ||
|---|---|---|
| Entry | Control Conditions | Conversion Yield (%) |
| I | 5 mg MnII3(tp)6/2(bpy)2.(dmf), light, acetonitrile (ACN) | 90 |
| II | 5 mg MnII3(tp)6/2(bpy)2.(dmf), light, no solvent | 2 |
| III | MnII3(tp)6/2(bpy)2.(dmf), no light, no heat | Nil |
| IV | MnII3(tp)6/2(bpy)2.(dmf), heat, no light | 2 |
| V | Heat, no light, no MnII3(tp)6/2(bpy)2.(dmf) | Nil |
| VI | light, no MnII3(tp)6/2(bpy)2.(dmf) | Nil |
| VII | 5 mg Mn2(tpa)2(dmf)2, light, no solvent | 9 |
| Reaction optimum conditions: 0.5 mmol (51 µL) benzylamine, 6.4 × 10−3 mmol photocatalyst, 2 mL acetonitrile (ACN), open air, irradiated for 24 h. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddig, L.A.; Alzard, R.H.; Abdelhamid, A.S.; Alzamly, A. Manganese-Based Metal-Organic Frameworks Photocatalysts for Visible Light-Driven Oxidative Coupling of Benzylamine under Atmospheric Oxygen: A Comparative Study. Catalysts 2023, 13, 613. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13030613
Siddig LA, Alzard RH, Abdelhamid AS, Alzamly A. Manganese-Based Metal-Organic Frameworks Photocatalysts for Visible Light-Driven Oxidative Coupling of Benzylamine under Atmospheric Oxygen: A Comparative Study. Catalysts. 2023; 13(3):613. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13030613
Chicago/Turabian StyleSiddig, Lamia A., Reem H. Alzard, Abdalla S. Abdelhamid, and Ahmed Alzamly. 2023. "Manganese-Based Metal-Organic Frameworks Photocatalysts for Visible Light-Driven Oxidative Coupling of Benzylamine under Atmospheric Oxygen: A Comparative Study" Catalysts 13, no. 3: 613. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13030613