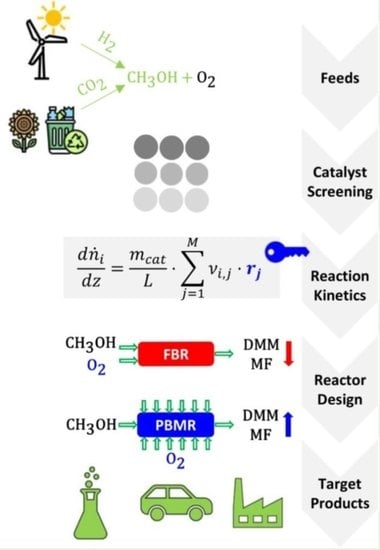
Selective Methanol Oxidation to Green Oxygenates—Catalyst Screening, Reaction Kinetics and Performance in Fixed-Bed and Membrane Reactors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental
2.1.1. Catalyst Screening
2.1.2. Kinetic Experiments
Influence of Residence Time
Influence of Oxygen Concentration
2.2. Reaction Kinetics and Reactor Concepts
2.2.1. Reaction Kinetics
Reaction Network Adaption
Parameter Estimation
2.2.2. Feasibility Study of Different Reactor Concepts and Dosing Strategies (FBR vs. PBMR)
3. Materials and Methods
3.1. Catalysts
3.2. Experimental
3.3. Mathematical Modeling
3.3.1. Parameter Estimation
3.3.2. Simulation Studies of FBR and PBMR
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Symbols
| adsorption energy | ||
| activation energy | ||
| molar flux | ||
| reaction rate constant | ||
| 0 | pre-exponential factor | |
| K | equilibrium constant of adsorption | |
| K0 | pre-exponential factor | |
| Keq | equilibrium constant of reaction | |
| reactor length | ||
| mass of catalyst | ||
| total molar flow rate | ||
| number of experiments | ||
| pressure | ||
| reaction rate | ||
| R | universal gas constant | |
| selectivity | ||
| temperature | ||
| total volumetric flow rate | ||
| molar fraction | ||
| conversion | ||
| yield | ||
| axial coordinate | ||
| Greek letters | ||
| enthalpy of reaction | ||
| entropy of reaction | ||
| - | performance indicator (conversion, selectivity, yield) | |
| - | stoichiometric coefficient | |
| - | Weisz-Prater criterion | |
| Subscripts | ||
| exp | experimental | |
| i | component index | |
| in | inlet | |
| j | reaction index | |
| sim | simulated | |
| ss | shell-side | |
| ts | tube-side | |
| Abbreviations | ||
| DMM | dimethoxymethane | |
| eq. | equation | |
| FBR | fixed-bed reactor | |
| MeOH | methanol | |
| MF | methyl formate | |
| OF | objective function | |
| O2 | oxygen | |
| PBMR | packed-bed membrane reactor | |
| ts/ss | tube-to-shell side feed ratio | |
| WHSV | weight hourly space velocity | |
References
- Ueckerdt, F.; Bauer, C.; Dirnaichner, A.; Everall, J.; Sacchi, R.; Luderer, G. Potential and risks of hydrogen-based e-fuels in climate change mitigation. Nat. Clim. Chang. 2021, 11, 384–393. [Google Scholar] [CrossRef]
- Ehmann, K.R.; Nisters, A.; Vorholt, A.J.; Leitner, W. Carbon Dioxide Hydrogenation to Formic Acid with Self-Separating Product and Recyclable Catalyst Phase. ChemCatChem 2022, 14. [Google Scholar] [CrossRef]
- Klemm, E.; Lobo, C.M.S.; Löwe, A.; Schallhart, V.; Renninger, S.; Waltersmann, L.; Costa, R.; Schulz, A.; Dietrich, R.-U.; Möltner, L.; et al. CHEMampere: Technologies for sustainable chemical production with renewable electricity and CO2, N2, O2, and H2O. Can. J. Chem. Eng. 2022. [Google Scholar] [CrossRef]
- Ott, J.; Gronemann, V.; Pontzen, F.; Fiedler, E.; Grossmann, G.; Kersebohm, D.B.; Weiss, G.; Witte, C. Methanol. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; ISBN 3527306730. [Google Scholar]
- Bertau, M.; Räuchle, K.; Offermanns, H. Methanol—Die Basischemikalie. Chem. Unserer Zeit 2015, 49, 312–329. [Google Scholar] [CrossRef]
- Oestreich, D.; Lautenschütz, L.; Arnold, U.; Sauer, J. Reaction kinetics and equilibrium parameters for the production of oxymethylene dimethyl ethers (OME) from methanol and formaldehyde. Chem. Eng. Sci. 2017, 163, 92–104. [Google Scholar] [CrossRef]
- Hackbarth, K.; Haltenort, P.; Arnold, U.; Sauer, J. Recent Progress in the Production, Application and Evaluation of Oxymethylene Ethers. Chem. Ing. Tech. 2018, 90, 1520–1528. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, Z.; Wang, G.; Dong, M.; Huang, L.; Wu, Z.; Fan, W.; Wang, J. Catalytic performance of V2O5/ZrO2–Al2O3 for methanol oxidation. Fuel 2013, 104, 22–27. [Google Scholar] [CrossRef]
- Zhao, H.; Bennici, S.; Cai, J.; Shen, J.; Auroux, A. Influence of the metal oxide support on the surface and catalytic properties of sulfated vanadia catalysts for selective oxidation of methanol. J. Catal. 2010, 274, 259–272. [Google Scholar] [CrossRef]
- Guo, H.; Chen, C.; Xiao, Y.; Wang, J.; Fan, Z.; Li, D.; Sun, Y. Influence of preparation method on the surface and catalytic properties of sulfated vanadia–titania catalysts for partial oxidation of methanol. Fuel Process. Technol. 2013, 106, 77–83. [Google Scholar] [CrossRef]
- Liu, H.; Iglesia, E. Selective oxidation of methanol and ethanol on supported ruthenium oxide clusters at low temperatures. J. Phys. Chem. B 2005, 109, 2155–2163. [Google Scholar] [CrossRef]
- Tatibouët, J.M. Methanol oxidation as a catalytic surface probe. Appl. Catal. A Gen. 1997, 148, 213–252. [Google Scholar] [CrossRef]
- Thavornprasert, K.; Capron, M.; Jalowiecki-Duhamel, L.; Gardoll, O.; Trentesaux, M.; Mamede, A.-S.; Fang, G.; Faye, J.; Touati, N.; Vezin, H.; et al. Highly productive iron molybdate mixed oxides and their relevant catalytic properties for direct synthesis of 1,1-dimethoxymethane from methanol. Appl. Catal. B Environ. 2014, 145, 126–135. [Google Scholar] [CrossRef]
- Thavornprasert, K.; Capron, M.; Jalowiecki-Duhamel, L.; Dumeignil, F. One-pot 1,1-dimethoxymethane synthesis from methanol: A promising pathway over bifunctional catalysts. Catal. Sci. Technol. 2016, 6, 958–970. [Google Scholar] [CrossRef]
- Jacob, E. C-1 Oxygenate als nachhaltige Kraftstoffe und deren günstige Eigenschaften. In Zukünftige Kraftstoffe; Maus, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 155–180. ISBN 978-3-662-58005-9. [Google Scholar]
- Kaiser, D.; Beckmann, L.; Walter, J.; Bertau, M. Conversion of Green Methanol to Methyl Formate. Catalysts 2021, 11, 869. [Google Scholar] [CrossRef]
- Benajes, J.; García, A.; Monsalve-Serrano, J.; Martínez-Boggio, S. Potential of using OMEx as substitute of diesel in the dual-fuel combustion mode to reduce the global CO2 emissions. Transp. Eng. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Martins, J.; Brito, F.P. Alternative Fuels for Internal Combustion Engines. Energies 2020, 13, 4086. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Popova, G.; Chesalov, Y.; Saraev, A.A.; Zemlyanov, D.Y.; Beloshapkin, S.A.; Knop-Gericke, A.; Schlögl, R.; Andrushkevich, T.V.; Bukhtiyarov, V.I. Selective oxidation of methanol to form dimethoxymethane and methyl formate over a monolayer V2O5/TiO2 catalyst. J. Catal. 2014, 311, 59–70. [Google Scholar] [CrossRef]
- Burger, J.; Siegert, M.; Ströfer, E.; Hasse, H. Poly(oxymethylene) dimethyl ethers as components of tailored diesel fuel: Properties, synthesis and purification concepts. Fuel 2010, 89, 3315–3319. [Google Scholar] [CrossRef]
- Maier, T.; Härtl, M.; Jacob, E.; Wachtmeister, G. Dimethyl carbonate (DMC) and Methyl Formate (MeFo): Emission characteristics of novel, clean and potentially CO2-neutral fuels including PMP and sub-23 nm nanoparticle-emission characteristics on a spark-ignition DI-engine. Fuel 2019, 256, 115925. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Song, H.; Wu, Q.; Zhao, Y.; Jiao, Q. Synthesis of methylal from methanol and formaldehyde catalyzed by Brønsted acid ionic liquids with different alkyl groups. RSC Adv. 2015, 5, 87200–87205. [Google Scholar] [CrossRef]
- Baranowski, C.J.; Bahmanpour, A.M.; Kröcher, O. Catalytic synthesis of polyoxymethylene dimethyl ethers (OME): A review. Appl. Catal. B Environ. 2017, 217, 407–420. [Google Scholar] [CrossRef]
- Bhatelia, T.; Lee, W.J.; Samanta, C.; Patel, J.; Bordoloi, A. Processes for the production of oxymethylene ethers: Promising synthetic diesel additives. Asia-Pac. J. Chem. Eng. 2017, 12, 827–837. [Google Scholar] [CrossRef]
- Verfahren zur Herstellung von Methylformiat durch Umsetzung von Methanol mit Kohlenmonoxid in Gegenwart eines Katalysatorsystems, das Alkaliformiat und Alkalialkoholat Enthält—European Patent Office—EP 2922815 B1. Available online: https://patentimages.storage.googleapis.com/4b/4e/85/35f7d91d1e7989/EP0427062B1.pdf (accessed on 8 March 2023).
- Rong, L.; Xu, Z.; Sun, J.; Guo, G. New methyl formate synthesis method: Coal to methyl formate. J. Energy Chem. 2018, 27, 238–242. [Google Scholar] [CrossRef]
- Reutemann, W.; Kieczka, H. Formic Acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; ISBN 3527306730. [Google Scholar]
- Lee, J.S.; Kim, J.C.; Kim, Y.G. Methyl formate as a new building block in C1 chemistry. Appl. Catal. 1990, 57, 1–30. [Google Scholar] [CrossRef]
- Niethammer, B.; Wodarz, S.; Betz, M.; Haltenort, P.; Oestreich, D.; Hackbarth, K.; Arnold, U.; Otto, T.; Sauer, J. Alternative Liquid Fuels from Renewable Resources. Chem. Ing. Tech. 2018, 90, 99–112. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, T.; Chen, S.; Zhao, Y.; Ma, X.; Gong, J. Selective oxidation of methanol to dimethoxymethane on V2O5–MoO3/γ-Al2O3 catalysts. Appl. Catal. B Environ. 2014, 160–161, 161–172. [Google Scholar] [CrossRef]
- Tatibouët, J.-M.; Lauron-Pernot, H. Transient isotopic study of methanol oxidation on unsupported V2O5. J. Mol. Catal. A Chem. 2001, 171, 205–216. [Google Scholar] [CrossRef]
- Sun, Q.; Fu, Y.; Liu, J.; Auroux, A.; Shen, J. Structural, acidic and redox properties of V2O5-TiO2-SO42− catalysts. Appl. Catal. A Gen. 2008, 334, 26–34. [Google Scholar] [CrossRef]
- Zhao, H.; Bennici, S.; Shen, J.; Auroux, A. Nature of surface sites of V2O5–TiO2/SO42− catalysts and reactivity in selective oxidation of methanol to dimethoxymethane. J. Catal. 2010, 272, 176–189. [Google Scholar] [CrossRef]
- Chen, S.; Meng, Y.; Zhao, Y.; Ma, X.; Gong, J. Selective Oxidation of Methanol to Dimethoxymethane over Mesoporous Al-P-V-O Catalysts. AIChE J. 2013, 59, 2587–2593. [Google Scholar] [CrossRef]
- Fan, Z.; Guo, H.; Fang, K.; Sun, Y. Efficient V2O5/TiO2 composite catalysts for dimethoxymethane synthesis from methanol selective oxidation. RSC Adv. 2015, 5, 24795–24802. [Google Scholar] [CrossRef]
- Chen, S.; Ma, X. The role of oxygen species in the selective oxidation of methanol to dimethoxymethane over VOx/TS-1 catalyst. J. Ind. Eng. Chem. 2017, 45, 296–300. [Google Scholar] [CrossRef]
- Tao, M.; Wang, H.; Bin Lu, B.L.; Zhao, J.; Cai, Q. Highly selective oxidation of methanol to dimethoxymethane over SO42−/V2O5–ZrO2. New J. Chem. 2017, 41, 8370–8376. [Google Scholar] [CrossRef]
- Sun, R.; Delidovich, I.; Palkovits, R. Dimethoxymethane as a Cleaner Synthetic Fuel: Synthetic Methods, Catalysts, and Reaction Mechanism. ACS Catal. 2019, 9, 1298–1318. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Popova, G.Y.; Chesalov, Y.A.; Saraev, A.A.; Andrushkevich, T.V.; Bukhtiyarov, V.I. Active component of supported vanadium catalysts in the selective oxidation of methanol. Kinet. Catal. 2016, 57, 82–94. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Song, F.; Zhang, Q.; Han, Y.; Tan, Y. Selective oxidation conversion of methanol/dimethyl ether. Chem. Commun. 2022, 58, 4687–4699. [Google Scholar] [CrossRef]
- Klose, F. Structure-Activity Relations of Supported Vanadia Catalysts and the Potential of Membrane Reactors for the Oxidative Dehydrogenation of Ethane, 1st ed.; Magdeburg, Univ., Fak. für Verfahrens- und Systemtechnik, Habil.-Schr.: Magdeburg, Germany, 2008; ISBN 978-3-939665-59-5. [Google Scholar]
- Walter, J.P. Selective Oxidation of Methanol to Green Oxygenates—Feasibility Study of Fixed-Bed and Membrane Reactors. Chem. Ing. Tech. 2023. [Google Scholar] [CrossRef]
- Broomhead, W.T.; Tian, W.; Herrera, J.E.; Chin, Y.-H.C. Kinetic Coupling of Redox and Acid Chemistry in Methanol Partial Oxidation on Vanadium Oxide Catalysts. ACS Catal. 2022, 12, 11801–11820. [Google Scholar] [CrossRef]
- Deshmukh, S.; van Annaland, M.S.; Kuipers, J. Kinetics of the partial oxidation of methanol over a Fe-Mo catalyst. Appl. Catal. A Gen. 2005, 289, 240–255. [Google Scholar] [CrossRef]
- Kapteijn, F.; van de Graaf, J.M.; Moulijn, J.A. One-component permeation maximum: Diagnostic tool for silicalite-1 membranes? AIChE J. 2000, 46, 1096–1100. [Google Scholar] [CrossRef]
- Yaws, C.L. The Yaws Handbook of Thermodynamic Properties for Hydrocarbons and Chemicals; Gulf: Houston, TX, USA, 2006; ISBN 1933762071. [Google Scholar]
- Ościk, J. Adsorption; Horwood, E., Ed.; Halsted Press: Chichester, NY, USA, 1982; ISBN 83-01-02568-9. [Google Scholar]
- Emig, G.; Klemm, E. Chemische Reaktionstechnik, 6th ed.; Springer Vieweg: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-49267-3. [Google Scholar]
- Hamel, C.; Tóta, Á.; Klose, F.; Tsotsas, E.; Seidel-Morgenstern, A. Analysis of single and multi-stage membrane reactors for the oxidation of short-chain alkanes—Simulation study and pilot scale experiments. Chem. Eng. Res. Des. 2008, 86, 753–764. [Google Scholar] [CrossRef]
- Walter, J.P.; Brune, A.; Seidel-Morgenstern, A.; Hamel, C. Model-based Analysis of Fixed-bed and Membrane Reactors of Various Scale. Chem. Ing. Tech. 2021, 93, 819–824. [Google Scholar] [CrossRef]
- Walter, J.P.; Brune, A.; Seidel-Morgenstern, A.; Hamel, C. Process Intensification of the Propane Dehydrogenation Considering Coke Formation, Catalyst Deactivation and Regeneration—Transient Modelling and Analysis of a Heat-Integrated Membrane Reactor. Catalysts 2021, 11, 1056. [Google Scholar] [CrossRef]
- Brune, A.; Wolff, T.; Seidel-Morgenstern, A.; Hamel, C. Analysis of Membrane Reactors for Integrated Coupling of Oxidative and Thermal Dehydrogenation of Propane. Chem. Ing. Tech. 2019, 91, 645–650. [Google Scholar] [CrossRef]
- Hamel, C.; Seidel-Morgenstern, A. Potenzial von Membranen zur verbesserten Reaktionsführung von Selektivoxidationen: Katalysator-, Reaktor- und Prozessebene. Chem. Ing. Tech. 2022, 94, 56–69. [Google Scholar] [CrossRef]
- Christiansen, J.A. The Elucidation of Reaction Mechanisms by the Method of Intermediates in Quasi-Stationary Concentrations; Elsevier: Amsterdam, The Netherlands, 1953; pp. 311–353. ISBN 9780120078059. [Google Scholar]
- Helfferich, F.G. Kinetics of Multistep Reactions (Comprehensive Chemical Kinetics, 0069-8040; v. 40); Elsevier Science Limited: Amsterdam, The Netherlands, 2004; ISBN 9780080473185. [Google Scholar]








| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Catalyst | Temperature [°C] | X(MeOH) [%] | S(DMM) [%] | S(MF) [%] | S(DME) [%] | S(FA) [%] | S(CO) [%] | Ymax(DMM) [%] |
| 1.4%VOx/Al2O3 | 150 | 16.4 | 26.3 | 16.5 | 57.2 | 0.0 | 0.0 | 4.3 |
| 15.7%VOx/ Al2O3 | 150 | 8.0 | 93.4 | 3.2 | 3.4 | 0.0 | 0.0 | 7.5 |
| 4.0%VOx/TiO2 | 200 | 15.9 | 24.5 | 12.4 | 5.6 | 57.5 | 0.0 | 3.9 |
| 6.6%VOx/TiO2 | 160 | 15.0 | 79.5 | 8.5 | 2.2 | 9.7 | 0.0 | 11.9 |
| 6.8%VOx/SiO2 | 240 | 25.1 | 24.4 | 11.3 | 4.9 | 59.4 | 0.0 | 6.1 |
| 15.9%VOx/SiO2 | 250 | 21.9 | 26.3 | 8.4 | 4.6 | 60.7 | 0.0 | 5.8 |
| (b) | ||||||||
| Catalyst | Temperature [°C] | X(MeOH) [%] | S(DMM) [%] | S(MF) [%] | S(DME) [%] | S(FA) [%] | S(CO) [%] | Ymax(MF) [%] |
| 1.4%VOx/Al2O3 | 150 | 16.4 | 26.3 | 16.5 | 57.2 | 0.0 | 0.0 | 2.7 |
| 15.7%VOx/ Al2O3 | 200 | 36.7 | 9.0 | 9.5 | 10.2 | 71.3 | 0.0 | 3.5 |
| 4.0%VOx/TiO2 | 250 | 67.8 | 0.6 | 8.5 | 2.9 | 83.7 | 4.3 | 5.8 |
| 6.6%VOx/TiO2 | 200 | 61.1 | 2.4 | 29.0 | 6.3 | 59.9 | 2.5 | 17.7 |
| 6.8%VOx/SiO2 | 280 | 80.3 | 1.4 | 5.6 | 3.0 | 87.8 | 2.2 | 4.5 |
| 15.9%VOx/SiO2 | 300 | 84.5 | 1.0 | 2.5 | 2.4 | 94.1 | 0.0 | 2.1 |
| Parameter | Value | Unit | Confidence Interval [%] |
|---|---|---|---|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walter, J.P.; Wolff, T.; Hamel, C. Selective Methanol Oxidation to Green Oxygenates—Catalyst Screening, Reaction Kinetics and Performance in Fixed-Bed and Membrane Reactors. Catalysts 2023, 13, 787. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13050787
Walter JP, Wolff T, Hamel C. Selective Methanol Oxidation to Green Oxygenates—Catalyst Screening, Reaction Kinetics and Performance in Fixed-Bed and Membrane Reactors. Catalysts. 2023; 13(5):787. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13050787
Chicago/Turabian StyleWalter, Jan P., Tanya Wolff, and Christof Hamel. 2023. "Selective Methanol Oxidation to Green Oxygenates—Catalyst Screening, Reaction Kinetics and Performance in Fixed-Bed and Membrane Reactors" Catalysts 13, no. 5: 787. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13050787