1. Introduction
Volatile organic compounds (VOCs) are gases that are emitted into the air from products or processes. Due to their properties, they are present in solvents, solvent-based paints and varnishes, glues, dispersants, degreasing agents, lubricants, and liquid fuels, and they are emitted from industries that synthesize them, generate them as by-products, or use them in their processes.
Due to their negative effects and widespread use, there is a clear need to avoid/reduce their emission into the environment. Worldwide, there are strict environmental regulations [
1,
2] that establish limits on the maximum concentrations of VOCs allowed to be vented into the air (the emission limit value (ELV)).
Catalytic oxidation is emerging as a promising technology for their reduction/elimination, particularly when: (a) the concentration of VOCs is relatively low and, therefore, recovery is economically unfeasible and (b) the concentration or flowrate of pollutants is not constant over time and, consequently, a versatile system capable of adapting to different temporal emission patterns is required [
3].
These end-of-pipe systems have a twofold objective: to keep the concentration below the ELV and to use the recovered heat to reduce the amount of fuel necessary to preheat large volumes of contaminated air up to the reaction temperature. This, in turn, serves a dual purpose: (i) to reduce the energy demand and, thus, the associated operating costs and (ii) to reduce the CO
2 emissions resulting from the use of fossil fuels in the removal process. Thus, the heat generated in the combustion of VOCs can be partially recovered through a feed-effluent heat exchanger (FEHE) to preheat the feed stream using the hot gases leaving the reactor. The heat needed to reach the reaction temperature can then be generated in a furnace where natural gas is admitted [
4].
Energy recovery introduces positive feedback structures into the system, which may dramatically alter the time constants of the plant [
5] and result in a variety of steady-state and dynamic phenomena, such as the snowball effect, extremely sluggish responses, oscillatory behaviour (limit cycles), and even instability [
5,
6,
7,
8].
Bildea et al. [
9] studied a coupled reactor/separation/recycle system for toluene hydrodealkylation (HDA) and found that the interaction between reaction and separation through material recycling can lead to unfeasibility, steady-state multiplicity, and instability.
Morud and Skogestad [
10] discussed the dynamics of an industrial multibed ammonia reactor where positive feedback due to heat integration led to oscillatory behaviour in the range from about 300 °C to 500 °C. The authors concluded that the physical cause for this somewhat unusual instability was a combination of the positive heat feedback in the preheater and the non-minimum phase behaviour (inverse response dynamics) of the reactor temperature response.
Luyben [
11] described the dynamic problems that occur in reactor–FEHE systems and showed that the positive feedback of energy can produce an open-loop unstable process. However, the system can be made closed-loop stable through the use of an inlet temperature controller that bypasses cold material around the heat exchanger and mixes it with the heated stream to achieve the desired inlet reactor temperature.
Additional units are usually included in heat-integrated designs as follows: (i) Heater for the start-up. Since positive feedback due to heat integration may lead to state multiplicity, the heater duty can be manipulated in a temperature control loop to ensure stable operation; (ii) Steam generator. The energy introduced by the heater has to be removed; for example, by increasing the steam. Since the furnace is a heat source and the excess energy is rejected to a heat sink (steam generator), the reactor can be viewed as a heat pump [
12].
Bildea and Dimian [
13] studied the steady-state and dynamic behaviour of a heat-integrated PFR consisting of a feed-effluent heat exchanger (FEHE), a furnace, an adiabatic tubular reactor, and a steam generator. The system exhibited oscillatory behaviour with realistic values for the model parameters, and the selection of the FEHE efficiency was a critical step to achieve the desired steady state and ensure stability. The research showed a close relationship between design and controllability.
Several control structures have been proposed to control the reactor inlet temperature. Silverstein and Shinnar [
14] studied the effects of design parameters on the dynamic stability of systems with an FEHE followed by a furnace before the adiabatic reactor. They recommended controlling the reactor inlet temperature with the furnace duty. They also explored bypassing cold material around the FEHE to provide an additional manipulated variable. For the HDA process, Terrill and Douglas [
15] examined the use of multiple FEHEs in series to preheat the reactor feed with hot reactor effluents. In the process, a furnace is located before the reactor. Among the most common control structures, the authors suggested the control of the temperature of the mixed stream after the FEHE through the manipulation of the bypass flow together with the control of the reactor inlet temperature through the manipulation of the fuel admitted to the furnace [
16]. If, as in the case of total closure of the bypass valve, the temperature rise in the FEHE is not sufficient, the fuel flow to the furnace could be manipulated for additional energy supply.
Luyben (2012) [
16] studied different control structure configurations for the production of dimethyl ether (DME) from methanol, an exothermic and reversible vapour-phase reaction. The author explored configurations without a furnace and with a furnace with different percentages of bypass stream. Finally, he proposed a novel flowsheet and control structure. Instead of mixing the cold bypass with the hot stream from the FEHE, the bypass was mixed with the stream coming from the furnace, and this mixed stream was, in turn, fed into the reactor. The stream passing through the FEHE was fed directly into the furnace. This setup permits tight control of the reactor inlet temperature, a key variable. In addition, a nonlinear feedforward (FF) control structure was employed to reject feed flowrate disturbances by manipulating the natural gas valve in order to reduce energy consumption in the furnace.
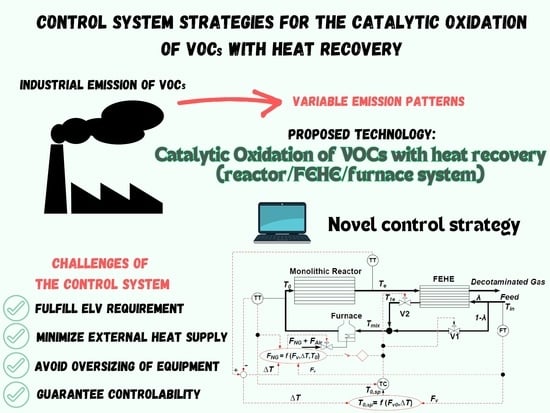
In the present study, the response of a reactor/FEHE/furnace system for the catalytic oxidation of VOCS under three different control system strategies to the two most frequent disturbances—changes in the process gas flowrate (FV0) and the feed VOC concentration (C0Et)—was evaluated.
Implementing an effective control strategy is crucial to (i) minimize out-of-specification periods (ELV requirement not fulfilled), (ii) avoid oversizing of the main equipment and reduce investment costs, (iii) minimize operating costs due to the external heat supply, and (iv) avoid severe thermal oscillations that can damage the catalyst and cause considerable stress on both the reactor and heat-exchanger materials.