α-NiS/g-C3N4 Nanocomposites for Photocatalytic Hydrogen Evolution and Degradation of Tetracycline Hydrochloride
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis, Structure, and Morphology
2.2. Optical and Photoelectrochemical Properties
2.3. Evaluation of Photocatalytic Performance
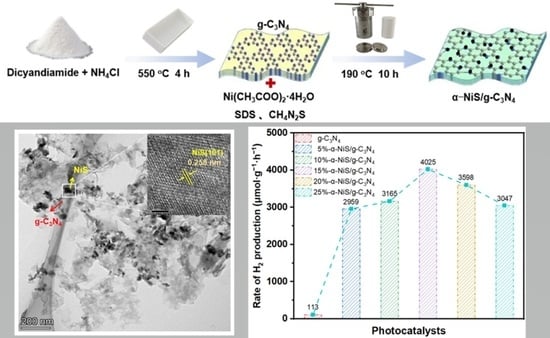
2.3.1. Photocatalytic H2 Generation and Stability of α-NiS/g-C3N4 Nanocomposites
2.3.2. Photocatalytic TC Degradation
2.4. Proposed Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Photocatalyst Preparation
3.2.1. Synthesis of g-C3N4
3.2.2. Synthesis of α-NiS/g-C3N4 Photocatalysts
3.3. Characterizations
3.4. Evaluation of Photoelectrochemical Performance
3.5. Evaluation of Photocatalytic Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Zhang, L.; Liu, J.; Zhou, J.; Jiao, Y.; Xiao, X.; Zhao, C.; Zhou, Y.; Ye, S.; Jiang, B.; et al. Porous Carbon Nitride Thin Strip: Precise Carbon Doping Regulating Delocalized Π-Electron Induces Elevated Photocatalytic Hydrogen Evolution. Small 2021, 17, 2006622. [Google Scholar] [CrossRef]
- Jang, D.; Choi, S.; Kwon, N.H.; Jang, K.Y.; Lee, S.; Lee, T.W.; Hwang, S.J.; Kim, H.; Kim, J.; Park, S. Water-Assisted Formation of Amine-Bridged Carbon Nitride: A Structural Insight into the Photocatalytic Performance for H2 Evolution under Visible Light. Appl. Catal. B Environ 2022, 310, 121313. [Google Scholar] [CrossRef]
- Ding, J.; Tang, Q.; Fu, Y.; Zhang, Y.; Hu, J.; Li, T.; Zhong, Q.; Fan, M.; Kung, H.H. Core-Shell Covalently Linked Graphitic Carbon Nitride-Melamine-Resorcinol-Formaldehyde Microsphere Polymers for Efficient Photocatalytic CO2 Reduction to Methanol. J. Am. Chem. Soc. 2022, 144, 9576–9585. [Google Scholar] [CrossRef]
- Lee, S.; Shin, E.Y.; Jang, D.; Choi, S.; Park, H.; Kim, J.; Park, S. Production of Mesoporous Carbon Nitrides and Their Photocatalytic Properties for Degradation of Organic Pollutants. Bull. Korean Chem. Soc. 2022, 43, 1124–1129. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Weng, B.; Lai, F.; Zhang, M.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A.; Long, J. Site-Sensitive Selective CO2 Photoreductionto CO over Gold Nanoparticles. Angew. Chem. Int. Ed. 2022, 61, e202204563. [Google Scholar] [CrossRef]
- Li, J.; Lv, X.; Weng, B.; Roeffaers, M.B.J.; Jia, H. Engineering light propagation for synergetic photo- and thermocatalysis toward volatile organic compounds elimination. Chem. Eng. J. 2023, 461, 142022. [Google Scholar] [CrossRef]
- Meng, S.; Chen, C.; Gu, X.; Wu, H.; Meng, Q.; Zhang, J.; Chen, S.; Fu, X.; Liu, D.; Lei, W. Efficient photocatalytic H2 evolution, CO2 reduction and N2 fixation coupled with organic synthesis by cocatalyst and vacancies engineering. Appl. Catal. B Environ. 2021, 285, 119789. [Google Scholar] [CrossRef]
- Liu, H.; Xu, C.; Li, D.; Jiang, H. Photocatalytic Hydrogen Production Coupled with Selective Benzylamine Oxidation over MOF Composites. Angew. Chem. Int. Ed. 2018, 57, 5379–5383. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, B.; Liu, Y.; Ren, Y.; Ma, J.; Zhou, X.; Cheng, X.; Deng, Y. Controllable Interface-Induced Co-Assembly toward Highly Ordered Mesoporous Pt@TiO2/g-C3N4 Heterojunctions with Enhanced Photocatalytic Performance. Adv. Funct. Mater. 2018, 28, 1806214. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Tan, L.; Cui, Z.; Jing, D.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; et al. Eradicating Multidrug-Resistant Bacteria Rapidly Using a Multi Functional g-C3N4@Bi2S3 Nanorod Heterojunction with or without Antibiotics. Adv. Funct. Mater. 2019, 29, 1900946. [Google Scholar] [CrossRef]
- Zada, A.; Humayun, M.; Raziq, F.; Zhang, X.; Qu, Y.; Bai, L.; Qin, C.; Jing, L.; Fu, H. Exceptional Visible-Light-Driven Cocatalyst-Free Photocatalytic Activity of g-C3N4 by Well Designed Nanocomposites with Plasmonic Au and SnO2. Adv. Energy Mater. 2016, 6, 1601190. [Google Scholar] [CrossRef]
- Feng, C.; Tang, L.; Deng, Y.; Wang, J.; Luo, J.; Liu, Y.; Ouyang, X.; Yang, H.; Yu, J.; Wang, J. Synthesis of Leaf-Vein-Like g-C3N4 with Tunable Band Structures and Charge Transfer Properties for Selective Photocatalytic H2O2 Evolution. Adv. Funct. Mater. 2020, 30, 2001922. [Google Scholar] [CrossRef]
- Wang, H.; Lin, Q.; Yin, L.; Yang, Y.; Qiu, Y.; Lu, C.; Yang, H. Biomimetic Design of Hollow Flower-Like g-C3N4@PDA Organic Framework Nanospheres for Realizing an Efficient Photoreactivity. Small 2019, 15, 1900011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.; Zhao, X.; Zhao, Y.; Tan, H.; Zhao, X.; Ma, Y.; Zhao, Z.; Song, S.; Wang, Y.; et al. Leaf-Mosaic-Inspired Vine-Like Graphitic Carbon Nitride Showing High Light Absorption and Efficient Photocatalytic Hydrogen Evolution. Adv. Energy Mater. 2018, 8, 1801139. [Google Scholar] [CrossRef]
- Yu, H.; Shang, L.; Bian, T.; Shi, R.; Waterhouse, G.I.N.; Zhao, Y.; Zhou, C.; Wu, L.Z.; Tung, C.H.; Zhang, T. Nitrogen-Doped Porous Carbon Nanosheets Templated from g-C3N4 as Metal-Free Electrocatalysts for Efficient Oxygen Reduction Reaction. Adv. Mater. 2016, 28, 5080–5086. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Qu, D.; An, L.; Jiang, W.; Wen, Y.; Wang, X.; Sun, Z. Highly Efficient P-Type Cu3P/n-Type g-C3N4 Photocatalyst through Z-Scheme Charge Transfer Route. Appl. Catal. B Environ 2019, 240, 253–261. [Google Scholar] [CrossRef]
- Luo, H.; Liu, Y.; Dimitrov, S.D.; Steier, L.; Guo, S.; Li, X.; Feng, J.; Xie, F.; Fang, Y.; Sapelkin, A.; et al. Pt Single-Atoms Supported on Nitrogen-Doped Carbon Dots for Highly Efficient Photocatalytic Hydrogen Generation. J. Mater. Chem. A 2020, 8, 14690–14696. [Google Scholar] [CrossRef]
- Wen, J.; Li, X.; Li, H.; Ma, S.; He, K.; Xu, Y.; Fang, Y.; Liu, W.; Gao, Q. Enhanced Visible-Light H2 Evolution of g-C3N4 Photocatalysts via the Synergetic Effect of Amorphous NiS and Cheap Metal-Free Carbon Black Nanoparticles as Co-Catalysts. Appl. Surf. Sci. 2015, 358, 204–212. [Google Scholar] [CrossRef]
- Guo, H.; Ke, Y.; Wang, D.; Lin, K.; Shen, R.; Chen, J.; Weng, W. Efficient Adsorption and Photocatalytic Degradation of Congo Red onto Hydrothermally Synthesized NiS Nanoparticles. J. Nanopart. Res. 2013, 15, 1475. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Jiang, Z.; Wei, W.; Chen, L.; Liu, D.; Qian, K.; Lü, X.; Xie, J. Fabrication of Noble-Metal-Free NiS2/g-C3N4 Hybrid Photocatalysts with Visible Light-Responsive Photocatalytic Activities. Res. Chem. Intermed. 2016, 42, 6483–6499. [Google Scholar] [CrossRef]
- Hou, Y.; Laursen, A.B.; Zhang, J.; Zhang, G.; Zhu, Y.; Wang, X.; Dahl, S.; Chorkendorff, I. Layered Nanojunctions for Hydrogen-Evolution Catalysis. Angew. Chem. Int. Edit. 2013, 125, 3709–3713. [Google Scholar] [CrossRef]
- Wen, M.Q.; Xiong, T.; Zang, Z.G.; Wei, W.; Tang, X.S.; Dong, F. Synthesis of MoS2/g-C3N4 Nanocomposites with Enhanced Visible-Light Photocatalytic Activity for the Removal of Nitric Oxide (NO). Opt. Express 2016, 24, 10205. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xiao, P.; Wang, P.; Yu, J. Amorphous molybdenum sulfide as highly efficient electron-cocatalyst for enhanced photocatalytic H2 evolution. Appl. Catal. B Environ. 2016, 193, 217–225. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, L.; Xie, J.; Chen, M. Ag2S/g-C3N4 Composite Photocatalysts for Efficient Pt-Free Hydrogen Production. The Co-Catalyst Function of Ag/Ag2S Formed by Simultaneous Photodeposition. Dalton Trans. 2014, 43, 4878–4885. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Luo, W.; Mo, Y.; Yu, H.; Cheng, B. In Situ Photodeposition of Amorphous CoSx on the TiO2 towards Hydrogen Evolution. Appl. Surf. Sci. 2018, 430, 448–456. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Cui, G.; Dong, Y.; Wang, G.; Jiang, P.; Wu, X.; Zhao, N. A Photochemical Synthesis Route to Typical Transition Metal Sulfides as Highly Efficient Cocatalyst for Hydrogen Evolution: From the Case of NiS/g-C3N4. Appl. Catal. B Environ. 2018, 225, 284–290. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M.; Ismail, A.A.; Bahnemann, D.W. Decoration of Mesoporous Graphite-like C3N4 Nanosheets by NiS Nanoparticle-Driven Visible Light for Hydrogen Evolution. Appl. Nanosci. 2018, 8, 1587–1596. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, J.; Wang, X.; Hong, X.; Fan, J.; Yu, H. Sulfur-Mediated Photodeposition Synthesis of NiS Cocatalyst for Boosting H2-Evolution Performance of g-C3N4 Photocatalyst. Chin. J. Catal. 2021, 42, 37–45. [Google Scholar] [CrossRef]
- Samaniego-Benitez, J.E.; Jimenez-Rangel, K.; Lartundo-Rojas, L.; García-García, A.; Mantilla, A. Enhanced Photocatalytic H2 Production over g-C3N4/NiS Hybrid Photocatalyst. Mater. Lett. 2021, 290, 129476. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; He, J. Precise Recognition of Zn(II) Ions by a Finely Designed Pair of α-NiS and β-NiS Nanostructures: A Sandwich Mode Recognition Approach. J. Environ. Chem. Eng. 2021, 9, 106837. [Google Scholar] [CrossRef]
- Surendran, S.; Vijaya Sankar, K.; John Berchmans, L.; Kalai Selvan, R. Polyol Synthesis of α-NiS Particles and Its Physico-Chemical Properties. Mater. Sci. Semicond. Process. 2015, 33, 16–23. [Google Scholar] [CrossRef]
- Shen, R.; Zhang, L.; Chen, X.; Jaroniec, M.; Li, N.; Li, X. Integrating 2D/2D CdS/α-Fe2O3 Ultrathin Bilayer Z-Scheme Heterojunction with Metallic β-NiS Nanosheet-Based Ohmic-Junction for Efficient Photocatalytic H2 Evolution. Appl. Catal. B Environ 2020, 266, 118619. [Google Scholar] [CrossRef]
- Shen, L.; Qi, S.; Jin, Y.; Li, C.; Cheng, J.; Wang, H.; Ma, H.; Li, L. α-NiS-β-NiS Growth on Cd0.5Zn0.5S Formed Schottky Heterojunctions for Enhanced Photocatalytic Hydrogen Production. New J. Chem. 2022, 46, 17469–17478. [Google Scholar] [CrossRef]
- Bi, Z.; Guo, R.; Ji, X.; Hu, X.; Wang, J.; Chen, X.; Pan, W. Direct Z-Scheme CoS/g-C3N4 Heterojunction with NiS Co-Catalyst for Efficient Photocatalytic Hydrogen Generation. Int. J. Hydrogen Energ. 2022, 47, 34430–34443. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Zhong, J.; Xu, H.; Yang, Y.; Vajtai, R.; Lou, J.; Liu, Y.; Du, D.; Li, H.; et al. Oxygenated Monolayer Carbon Nitride for Excellent Photocatalytic Hydrogen Evolution and External Quantum Efficiency. Nano Energy 2016, 27, 138–146. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced Photoresponsive Ultrathin Graphitic-Phase C3N4 Nanosheets for Bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef]
- Saeed, M.; Haq Aul Muneer, M.; Ahmad, A.; Bokhari, T.H.; Sadi, Q. Synthesis and characterization of Bi2O3 and Ag-Bi2O3 and evaluation of their photocatalytic activities towards photodegradation of crystal violet dye. Phys. Scr. 2021, 96, 125707. [Google Scholar] [CrossRef]
- Mo, Z.; Xu, H.; Chen, Z.; She, X.; Song, Y.; Lian, J.; Zhu, X.; Yan, P.; Lei, Y.; Yuan, S.; et al. Construction of MnO2/Monolayer g-C3N4 with Mn Vacancies for Z-Scheme Overall Water Splitting. Appl. Catal. B Environ. 2019, 241, 452–460. [Google Scholar] [CrossRef]
- Xu, Y.; Du, C.; Zhou, C.; Yang, S. Ternary Noble-Metal-Free Heterostructured NiS-CuS-C3N4 with near-Infrared Response for Enhanced Photocatalytic Hydrogen Evolution. Int. J. Hydrogen Energy 2020, 45, 4084–4094. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, K.; Sun, R.; Ma, J. Biorefinery-Assisted Ultra-High Hydrogen Evolution via Metal-Free Black Phosphorus Sensitized Carbon Nitride Photocatalysis. Chem. Eng. J. 2022, 446, 137128. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, J.; Zhou, W.; Li, Y.; Li, X.; Zhang, S.; Fan, J.; Lv, K. Synergistic Effects of Holey Nanosheet and Sulfur-Doping on the Photocatalytic Activity of Carbon Nitride towards NO Removal. Chemosphere 2023, 316, 137813. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Deng, M.; Hou, D.; Huang, L.; Qiao, X.; Li, D. An Amorphous NiSx Film as a Robust Cocatalyst for Boosting Photocatalytic Hydrogen Generation over Ultrafine ZnCdS Nanoparticles. Mater. Adv. 2021, 2, 3881–3891. [Google Scholar] [CrossRef]
- Zhang, K.; Mou, Z.; Cao, S.; Wu, S.; Xu, X.; Li, C. Well-Designed NiS/CdS Nanoparticles Heterojunction for Efficient Visible-Light Photocatalytic H2 Evolution. Int. J. Hydrogen Energy 2022, 47, 12605–12614. [Google Scholar] [CrossRef]
- Xu, J.; Qi, Y.; Wang, L. In Situ Derived Ni2P/Ni Encapsulated in Carbon/g-C3N4 Hybrids from Metal-Organic Frameworks/g-C3N4 for Efficient Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ 2019, 246, 72–81. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, C.; Ma, H.; Li, G.; Chen, L.; Sun, Y.; Chen, Z.; Fang, P.; Fu, Q.; Pan, C. Synthesis of Flower-Liked Twin Crystal Ternary Ni/NiS/Zn0.2Cd0.8S Catalyst for Highly Efficient Hydrogen Production. Chem. Eng. J. 2021, 406, 126878. [Google Scholar] [CrossRef]
- Yasin, M.; Saeed, M.; Muneer, M.; Usman, M.; Haq A ul Sadia, M.; Altaf, M. Development of Bi2O3-ZnO heterostructure for enhanced photodegradation of rhodamine B and reactive yellow dyes. Surf. Interfaces 2022, 30, 101846. [Google Scholar] [CrossRef]
- Saeed, M.; Khan, I.; Adeel, M.; Akram, N.; Muneer, M. Synthesis of a CoO-ZnO photocatalyst for enhanced visible-light assisted photodegradation of methylene blue. New J. Chem. 2022, 46, 2224. [Google Scholar] [CrossRef]
- Nong, J.; Jin, Y.; Tan, J.; Ma, H.; Lian, Y. Construction of NiCo-LDH/g-C3N4 Heterojunctions as Efficient Photocatalysts for Enhanced Degradation of Tetracycline Hydrochloride and Hydrogen Evolution. New J. Chem. 2022, 46, 22830–22840. [Google Scholar] [CrossRef]
- Wang, W.; Niu, Q.; Zeng, G.; Zhang, C.; Huang, D.; Shao, B.; Zhou, C.; Yang, Y.; Liu, Y.; Guo, H.; et al. 1D porous tubular g-C3N4 capture black phosphorus quantum dots as 1D/0D metal-free photocatalysts for oxytetracycline hydrochloride degradation and hexavalent chromium reduction. Appl. Catal. B Environ. 2020, 273, 119051. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, Z.; Xia, Y.; Yin, W.; Hou, J.; Zhu, X.; Yi, J.; Wang, S.; Ning, X.; Wang, X. Synchronous synthesis of S-doped carbon nitride/nickel sulfide photocatalysts for efficient dye degradation and hydrogen evolution. Appl. Surf. Sci. 2023, 608, 154974. [Google Scholar] [CrossRef]
- Wang, R.; Yang, P.; Wang, S.; Wang, X. Distorted Carbon Nitride Nanosheets with Activated n ⟶ Π* Transition and Preferred Textural Properties for Photocatalytic CO2 Reduction. J. Catal. 2021, 402, 166–176. [Google Scholar] [CrossRef]
- Su, B.; Huang, H.; Ding, Z.; Roeffaers, M.B.J.; Wang, S.; Long, J. S-Scheme CoTiO3/Cd9.51Zn0.49S10 Heterostructures for Visible-Light Driven Photocatalytic CO2 Reduction. J. Mater. Sci. Technol. 2022, 124, 164–170. [Google Scholar] [CrossRef]
- Gao, X.; Zeng, D.; Yang, J.; Ong, W.-J.; Fujita, T.; He, X.; Liu, J.; Wei, Y. Ultrathin Ni(OH)2 Nanosheets Decorated with Zn0.5Cd0.5S Nanoparticles as 2D/0D Heterojunctions for Highly Enhanced Visible Light-Driven Photocatalytic Hydrogen Evolution. Chin. J. Catal. 2021, 42, 1137–1146. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Lv, H.; Cao, Y.; Ren, H. Boosting the photocatalytic hydrogen evolution activity of g-C3N4 nanosheets by Cu2(OH)2CO3-modification and dye-sensitization. Dalton T. 2019, 48, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, X.; Luo, H.; Su, B.; Ma, J. Facile Synthesis of Ni-Based Layered Double Hydroxides with Superior Photocatalytic Performance for Tetracycline Antibiotic Degradation. J. Solid State Chem. 2022, 307, 122827. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Xiao, Z.; Liu, S.; Hu, J.; Long, X.; Wu, L.; Sun, C.; Chen, K.; Jiao, F. Construction of Z-Scheme Heterojunction of (BiO)2CO3/ZnFe-LDH for Enhanced Photocatalytic Degradation of Tetracycline. J. Alloys Compd. 2022, 900, 163450. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.; Su, B.; Ma, J. Hydrothermal Construction of Flower-like g-C3N4/NiZnAl-LDH S-Scheme Heterojunction with Oxygen Vacancies for Enhanced Visible-Light Triggered Photocatalytic Performance. J. Alloys Compd. 2022, 922, 166098. [Google Scholar] [CrossRef]
- Liu, H.Y.; Niu, C.G.; Guo, H.; Liang, C.; Huang, D.W.; Zhang, L.; Yang, Y.Y.; Li, L. In Suit Constructing 2D/1D MgIn2S4/CdS Heterojunction System with Enhanced Photocatalytic Activity towards Treatment of Wastewater and H2 Production. J. Colloid Interface Sci. 2020, 576, 264–279. [Google Scholar] [CrossRef]
- Liu, H.Y.; Niu, C.G.; Guo, H.; Huang, D.W.; Liang, C.; Yang, Y.Y.; Tang, N.; Zhang, X.G. Integrating the Z-Scheme Heterojunction and Hot Electrons Injection into a Plasmonic-Based Zn2In2S5/W18O49 Composite Induced Improved Molecular Oxygen Activation for Photocatalytic Degradation and Antibacterial Performance. J. Colloid Interface Sci. 2022, 610, 953–969. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Wang, C.; Shen, L.; Wang, H.; Lian, Y.; Zhang, H.; Ma, H.; Zhang, Y.; Zhang, J.Z. α-NiS/g-C3N4 Nanocomposites for Photocatalytic Hydrogen Evolution and Degradation of Tetracycline Hydrochloride. Catalysts 2023, 13, 983. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13060983
Qi H, Wang C, Shen L, Wang H, Lian Y, Zhang H, Ma H, Zhang Y, Zhang JZ. α-NiS/g-C3N4 Nanocomposites for Photocatalytic Hydrogen Evolution and Degradation of Tetracycline Hydrochloride. Catalysts. 2023; 13(6):983. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13060983
Chicago/Turabian StyleQi, Huajin, Chenyu Wang, Luping Shen, Hongmei Wang, Yuan Lian, Huanxia Zhang, Hongxia Ma, Yong Zhang, and Jin Zhong Zhang. 2023. "α-NiS/g-C3N4 Nanocomposites for Photocatalytic Hydrogen Evolution and Degradation of Tetracycline Hydrochloride" Catalysts 13, no. 6: 983. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13060983