Pretreatment and Nanoparticles as Catalysts for Biogas Production Reactions in Pepper Waste and Pig Manure
Abstract
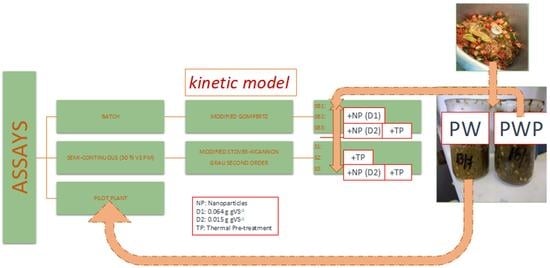
:1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Raw Materials
2.2. Biochemical Methane Potential (BMP) of Different Strategies with PWP
2.3. Different Pretreatment for Assays Semi-Continuous with PM and PWP
2.4. Effect of OLR on Different Parameters
2.5. Digestate Pilot Plant Experiment
3. Materials and Methods
3.1. Evaluated Raw Materials
3.2. Digester Used and Experimental Design
3.3. Analytical Methods
3.4. Evaluation of Substrate Removal Kinetic Models
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Union. Directiva (UE) 2018/2001 del Parlamento Europeo y del Consejo de 11 de Diciembre de 2018 Relativa al Fomento del Uso de Energía Procedente de Fuentes Renovables. In Diario Oficial de la Unión Europea; European Union: Strasbourg, France, 2018. [Google Scholar]
- Manual Sobre las Biorrefinerías en España; Ministerio de Economía, Industria y Competitividad: Madrid, Spain, 2017.
- Consejería de Economía, Ciencia y Agenda Digital. VII Plan Regional de Investigación, Desarrollo Tecnológico e Investigación de Extremadura 2022–2025; Consejería de Economía, Ciencia y Agenda Digital: Mérida, Spain, 2022. [Google Scholar]
- Instituto Nacional de Estadística. Available online: https://www.ine.es/jaxi/Datos.htm?path=/t26/p067/p02/residuos/serie/l0/&file=02001.px (accessed on 3 January 2023).
- Ministerio de Agricultura, Pesca y Alimentación. Cifras del Sector de Frutas y Hortalizas; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2022.
- Available online: https://es.statista.com/estadisticas/510920/produccion-de-pimiento-fresco-en-españa-por-comunidad-autonoma/ (accessed on 3 June 2023).
- Ros, M.; Pascual, J.A.; Ayuso, M.; Morales, A.B.; Miralles, J.R.; Solera, C. Salidas valorizables de los residuos y subproductos orgánicos de la industria de los transformados de frutas y hortalizas: Proyecto LIFE + Agrowaste. Residuos Rev. Técnica 2012, 22, 28–35. [Google Scholar]
- Vulic, J.; Seregelj, V.; Kalusevic, A.; Levic, S.; Nedovic, V.; Saponjac, V.T.; Canadanovic-Brunet, J.; Cetkovic, G. Bioavailability and bioactivity of encapsulated phenolics and carotenoids isolated from red pepper wastes. Molecules 2019, 24, 2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mórtola, N.; Romaniuk, R.; Consentino, V.; Eiza, M.; Carfagno, P.; Rizzo, P.; Bres, P.; Riera, N.; Roba, M.; Butti, M.; et al. Potential Use of a Poultry Manure Digestate as a Biofertiliser: Evaluation of Soil Properties and Lactuca sativa Growth. Pedosphere 2019, 29, 60–69. [Google Scholar] [CrossRef]
- Riaño, B.; Molinuevo-Salcés, B.; Parralejo, A.; Royano, L.; González, J.; García-González, M.C. Techno-economic evaluation of anaerobic co-digestion fo pepper waste and swine manure. Biomass Convers. Biorefin. 2021, 13, 7763–7774. [Google Scholar] [CrossRef]
- Li, R.; Tan, W.; Zhao, X.; Dang, Q.; Song, Q.; Xi, B.; Zhang, X. Evaluation on the Methane Production Potential of Wood Waste Pretreated with NaOH and Co-Digested with Pig Manure. Catalysts 2019, 9, 539. [Google Scholar] [CrossRef] [Green Version]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Femi Bakare, B. Application of Bioelectrochemical System and Magnetite Nanoparticles on the Anaerobic Digestion of Sewage Sludge: Effect of Electrode Configuration. Catalysts 2022, 12, 642. [Google Scholar] [CrossRef]
- Goswami, L.; Kushwaha, A.; Singh, A.; Saha, P.; Choi, Y.; Maharana, M.; Patil, S.V.; Kim, B.S. Nano-Biochar as a Sustainable Catalyst for Anaerobic Digestion: A Synergetic Closed-Loop Approach. Catalysts 2022, 12, 186. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S. Kinetics and Nanoparticle Catalytic Enhancement of Biogas Production from Wastewater Using a Magnetized Biochemical Methane Potential (MBMP) System. Catalysts 2020, 10, 1200. [Google Scholar] [CrossRef]
- Somridhivej, B.; Boyd, C.E. An assessment of factors affecting the reliability of total alkalinity measurements. Aquaculture 2016, 459, 99–109. [Google Scholar] [CrossRef]
- Flotats, X.; Bonmatí, A.; Campos, E.; Antúnez, M. Ensayos en discontinuo de co-digestión anaerobia termofílica de purines de cerdo y lodos residuales. Inf. Tecnol. 1999, 10, 79–85. [Google Scholar]
- Zhang, W.; Kong, T.; Xing, W.; Li, R.; Yang, T.; Yao, N.; Lv, D. Links between carbon/nitrogen ratio, synergy and microbial characteristics of long-term semi-continuous anaerobic co-digestion of food waste, cattle manure and corn straw. Bioresour. Technol. 2022, 343, 126094. [Google Scholar] [CrossRef]
- Xiao, Y.; Zan, F.; Zhang, W.; Hao, T. Alleviating nutrient imbalance of low carbon-to-nitrogen ratio food waste in anaerobic digestion by controlling the inoculum-to-substrate ratio. Bioresour. Technol. 2022, 346, 126342. [Google Scholar] [CrossRef]
- Slopiecka, K.; Liberti, F.; Massoli, S.; Bartocci, P.; Fantozzi, F. Chemical and physical characterization of food waste to improve its use in anaerobic digestion plants. Energy Nexus 2022, 5, 100049. [Google Scholar] [CrossRef]
- Samoraj, M.; Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Szopa, D.; Moustakas, K.; Chojnacka, K. The challenges and perspectives for anaerobic digestion of animal waste and fertilizer application of the digestate. Chemosphere 2022, 295, 133799. [Google Scholar] [CrossRef]
- Arhoun, B.; Villen-Guzman, M.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M.; Garcia-Herruzo, F.; Vereda-Alonso, C. Anaerobic co-digestion of mixed sewage sludge and fruits and vegetable wholesale market waste: Composition and seasonality effect. J. Water Process Eng. 2019, 31, 100848. [Google Scholar] [CrossRef]
- Gallego, L.M.; Portillo, E.; Navarrete, B.; González, R. Estimation of methane production through the anaerobic digestion of greenhouse horticultural waste: A real case study for Almeria region. Sci. Total Environ. 2022, 807, 151012. [Google Scholar] [CrossRef]
- González, J.F.; Parralejo, A.I.; González, J.; Álvarez, A.; Sabio, E. Optimization of the production and quality of biogas in the anaerobic digestion of different types of biomass in a batch laboratory biodigester and pilot plant: Numerical modeling, kinetic study and hydrogen potential. Int. J. Hydrogen Energy 2022, 43, 39386–39403. [Google Scholar] [CrossRef]
- Chiappero, M.; Cillerai, F.; Berruti, F.; Masek, O.; Fiore, S. Addition of Different Biochars as Catalysts during the Mesophilic Anaerobic Digestion of Mixed Wastewater Sludge. Catalysts 2021, 11, 1094. [Google Scholar] [CrossRef]
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. The effect of Fe2NiO4 and Fe2NiO4Zn magnetic nanoparticles on anaerobic digestion activity. Sci. Total Environ. 2018, 642, 276–284. [Google Scholar] [CrossRef]
- De la Lama, C.; Borja, R.; Rincón, B. Performance evaluation and substrate removal kinetics in the semi-continuous anaerobic digestion of thermally pretreated two phase olive pomace or “Alperujo”. Process Saf. Environ. Prot. 2017, 105, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Parralejo, A.I.; Royano, L.; Cabanillas, J.; González, J. Biogas from Nitrogen-Rich Biomass as an Alternative to Animal Manure Co-Substrate in Anaerobic Co-Digestion Processes. Enegies 2022, 15, 5978. [Google Scholar] [CrossRef]
- Drosg, B. Process Monitoring in Biogas Plant; Task 37—Energy from Biogas and Landfill Gas; International Energy Agency (IEA) Bioenergy: Paris, France, 2017. [Google Scholar]
- RD 535/2017; Sobre Productos Fertilizantes de 26 de Mayo. Ministerio de la Presidencia y para las Administraciones Territoriales: Madrid, Spain, 2017.
- Agriculture & Horticulture Development Board (AHDB). Nutrient Management Guide (RB209); Section 2: Organic Materials; Agriculture & Horticulture Development Board (AHDB): Kenilworth, UK, 2017; p. 35. [Google Scholar]
- APHA. Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- EPA-600/4-79-020; Methods for Chemical Analysis of Water and Wastes. Environmental Protection Agency: Washington, DC, USA; Environmental Monitoring and Support Laboratory: Des Plaines, IL, USA, 1983.
- Buchauer, K. Titrations verfahren in der Abwasserund Schlam-manalytik zur Bestimmung von flüchtigen organischen Säuren. Das Gas- und Wasserfach (gfw). Wasser Abwasser 1997, 138, 313–320. [Google Scholar]
- Norma UNE-EN 16948; Biocombustibles Sólidos, Determinación de Contenido Total de C, H y N, Método Instrumental. University of New England: Biddeford, UK, 2015.








| Parameter | PM | PW | PWP |
|---|---|---|---|
| pH | 7.70 ± 0.10 | 4.18 ± 0.04 | 4.10 ± 0.29 |
| Redox potential, mV | −362 ± 23 | −100 ± 1 | 206 ± 10 |
| Alkalinity, mg CaCO3 L−1 | 9379 ± 75 | - | - |
| N-NH4, mg L−1 | 1860 ± 85 | 870 ± 30 | 390 ± 80 |
| C, % | 2.23 ± 0.30 | 7.12 ± 0.19 | 7.63 ± 1.85 |
| N, % | 0.30 ± 0.03 | 0.36 ± 0.01 | 0.42 ± 0.09 |
| C/N | 7.32 ± 0.36 | 18.04 ± 1.37 | 18.71 ± 2.17 |
| TS, % | 5.71 ± 0.02 | 13.31 ± 0.29 | 17.17 ± 3.33 |
| VS *, % | 3.98 ± 0.12 | 12.32 ± 0.23 | 15.80 ± 3.14 |
| Ca, ppm | 2663 ± 48 | 1003 ± 21 | 1965 ± 18 |
| Fe, ppm | 209 ± 2 | 76 ± 1 | 120 ± 2 |
| K, ppm | 240 ± 30 | 2317 ± 25 | 1953 ± 4 |
| Mg, ppm | 1208 ± 11 | 439 ± 1 | 617 ± 4 |
| Na, ppm | 913 ± 2 | 64 ± 1 | 262 ± 3 |
| P, ppm | 1562 ± 7 | 473 ± 7 | 807 ± 46 |
| Al, ppm | 152 ± 7 | 81 ± 1 | 95 ± 5 |
| Parameter | SB1 | SB2 | SB3 | |||
|---|---|---|---|---|---|---|
| Methane average yield, NL kg VS−1 | 464 ± 25 | 331 ± 57 | 364 ± 49 | |||
| Methane average concentration, % | 59 ± 2 | 56 ± 6 | 60 ± 1 | |||
| Replicates | R1 | R2 | R1 | R2 | R1 | R2 |
| Rmax, Nm3 kg VS−1 d−1 | 0.64 | 0.75 | 0.82 | 1.51 | 0.85 | 1.00 |
| l, d | 2.82 | 1.56 | 0.77 | 1.29 | - | - |
| R2 | 0.9888 | 0.9757 | 0.9387 | 0.9727 | 0.9686 | 0.9766 |
| Period | Period I | Period II | Period III |
|---|---|---|---|
| S1 | |||
| Methane yield, NL kg VS−1 | 139 | 149 | 149 |
| kB, g COD L−1 | 4.42 | ||
| Umax, d−1 | 1.03 | ||
| k2, d−1 | 0.73 | ||
| S2 | |||
| Methane yield, NL kg VS−1 | 158 | 160 | 156 |
| kB, g COD L−1 | 4.98 | ||
| Umax, d−1 | 1.49 | ||
| k2, d−1 | 0.90 | ||
| S3 | |||
| Methane yield, NL kg VS−1 | 153 | 156 | 155 |
| kB, g COD L−1 | 4.33 | ||
| Umax, d−1 | 1.31 | ||
| k2, d−1 | 0.64 | ||
| Parameter | Period I | Period II |
|---|---|---|
| pH | 7.91 ± 0.10 | 7.95 ± 0.05 |
| Redox potential, mV | −388 ± 12 | −409 ± 9 |
| Alkalinity, mg CaCO3 L−1 | 10,892 ± 2 | 10,340 ± 37 |
| Methane yield, NL kg VS−1 | 173 ± 45 | 264 ± 55 |
| C, % | 1.06 ± 0.03 | 2.20 ± 0.20 |
| N, % | 0.21 ± 0.01 | 0.37 ± 0.02 |
| C/N | 4.98 ± 0.15 | 6.02 ± 1.14 |
| Ca, ppm | 779 ± 4 | 2259 ± 9 |
| Fe, ppm | 75 ± 2 | 230 ± 2 |
| K, ppm | 1317 ± 28 | 2467 ± 29 |
| Mg, ppm | 526 ± 2 | 1121 ± 3 |
| Na, ppm | 669 ± 2 | 596 ± 6 |
| P, ppm | 359 ± 10 | 1052 ± 15 |
| Al, ppm | 40 ± 2 | 157 ± 2 |
| Zn, ppm | 34 ± 1 | 141 ± 1 |
| Cu, ppm | 10 ± 1 | 38 ± 1 |
| Cr, ppm | <5 | <5 |
| Ni, ppm | <5 | <5 |
| Parameter | Period I | Period II |
|---|---|---|
| Assimilable N content, % | 0.12 | 0.20 |
| Assimilable P2O5 content, % | 0.80 | 2.33 |
| Assimilable K2O content, % | 1.01 | 1.89 |
| Fertilizer classification [28] | A | A |
| Assay | OLR, g VS LD−1 d−1 | Mixture Composition/Feed | Hydraulic Retention Time (HRT), d |
|---|---|---|---|
| SB1 | - | Inoculum–PWP (ratio: 1:2) | - |
| SB2 | - | Inoculum–PWP (ratio: 1:2) with nanoparticles (0.064 g g VS−1) | - |
| SB3 | - | Inoculum–PWP (ratio: 1:2) with nanoparticles (0.015 g g VS−1) and thermal pretreatment | - |
| S1 | Period I: 1.26 | 50% PM and 50% PWP (on VS basis) | 28 |
| Period II: 1.47 | 24 | ||
| Period III: 1.88 | 19 | ||
| S2 | Period I: 1.26 | 50% PM and 50% PWP (on VS basis) with thermal pretreatment | 28 |
| Period II: 1.47 | 24 | ||
| Period III: 1.88 | 19 | ||
| S3 | Period I: 1.26 | 50% PM and 50% PWP (on VS basis) with thermal pretreatment and nanoparticles (0.015 g g VS−1) | 28 |
| Period II: 1.47 | 24 | ||
| Period III: 1.88 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parralejo Alcobendas, A.I.; Royano Barroso, L.; Cabanillas Patilla, J.; González Cortés, J. Pretreatment and Nanoparticles as Catalysts for Biogas Production Reactions in Pepper Waste and Pig Manure. Catalysts 2023, 13, 1029. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13071029
Parralejo Alcobendas AI, Royano Barroso L, Cabanillas Patilla J, González Cortés J. Pretreatment and Nanoparticles as Catalysts for Biogas Production Reactions in Pepper Waste and Pig Manure. Catalysts. 2023; 13(7):1029. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13071029
Chicago/Turabian StyleParralejo Alcobendas, Ana Isabel, Luis Royano Barroso, Juan Cabanillas Patilla, and Jerónimo González Cortés. 2023. "Pretreatment and Nanoparticles as Catalysts for Biogas Production Reactions in Pepper Waste and Pig Manure" Catalysts 13, no. 7: 1029. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13071029