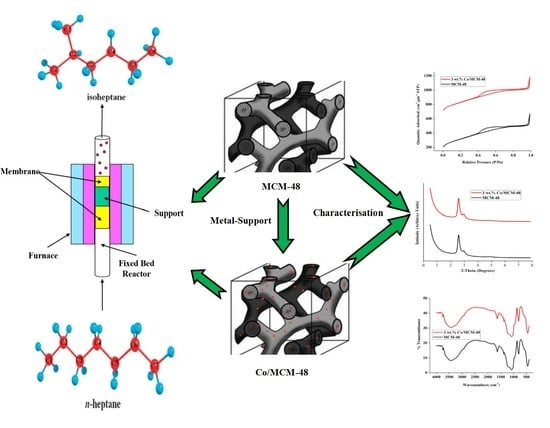
Utilization of Loaded Cobalt onto MCM-48 Mesoporous Catalyst as a Heterogeneous Reaction in a Fixed Bed Membrane Reactor to Produce Isomerization Product from n-Heptane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Conversion of n-Heptane
2.3. Selectivity to Isomerization
2.4. Selectivity to Cracking
3. Experimental
3.1. Chemicals
3.2. Synthesis of MCM-48
3.3. Loading of Metals onto MCM-48
3.4. Preparation of Disk MCM-48 Membranes
3.5. Characterization
3.6. Catalytic Test and Isoheptane Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saracco, G.; Neomagus, H.W.J.P.; Versteeg, G.F.; van Swaaij, W.P.M. High-temperature membrane reactors: Potential and problems. Chem. Eng. Sci. 1999, 54, 1997–2017. [Google Scholar] [CrossRef] [Green Version]
- Julbe, A.; Farrusseng, D.; Guizard, C. Porous ceramic membranes for catalytic reactors—Overview and new ideas. J. Membr. Sci. 2001, 181, 3–20. [Google Scholar] [CrossRef]
- Westermann, T.; Melin, T. Flow-through catalytic membrane reactors—Principles and applications. Chem. Eng. Process. Process Intensif. 2009, 48, 17–28. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.; Wang, C.; Lv, G.; Wang, D.; Ma, H.; Tian, Z. Direct synthesis of shaped MgAPO-11 molecular sieves and the catalytic performance in n-dodecane hydroisomerization. RSC Adv. 2021, 11, 25364–25374. [Google Scholar] [CrossRef] [PubMed]
- Jaroszewska, K.; Fedyna, M.; Trawczyński, J. Hydroisomerization of long-chain n-alkanes over Pt/AlSBA-15+zeolite bimodal catalysts. Appl. Catal. B Environ. 2019, 255, 117756. [Google Scholar] [CrossRef]
- Al-Shafei, E.N.; Albahar, M.Z.; Aljishi, M.F.; Akah, A.; Aljishi, A.N.; Alasseel, A. Catalytic conversion of heavy naphtha to reformate over the phosphorus-ZSM-5 catalyst at a lower reforming temperature. RSC Adv. 2022, 12, 25465–25477. [Google Scholar] [CrossRef]
- Oloye, F.F.; Aliyev, R.; Anderson, J.A. Hydroisomerisation of n-heptane over Pt/sulfated zirconia catalyst at atmospheric pressure. Fuel 2018, 222, 569–573. [Google Scholar] [CrossRef]
- Al-Shathr, A.; Shakor, Z.M.; Majdi, H.S.; AbdulRazak, A.A.; Albayati, T.M. Comparison between Artificial Neural Network and Rigorous Mathematical Model in Simulation of Industrial Heavy Naphtha Reforming Process. Catalysts 2021, 11, 1034. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, Y.; Si, M.; Wei, Q.; Zhou, Y. Hydroisomerization of n-Hexadecane Over Nickel-Modified SAPO-11 Molecular Sieve-Supported NiWS Catalysts: Effects of Modification Methods. Front. Chem. 2022, 10, 857473. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Z.; Sun, Y.; Jiang, S.; Tian, J. Optimization of the Countercurrent Continuous Reforming Process Based on Equation-Oriented Modeling and the SQP Algorithm. ACS Omega 2022, 7, 1757–1771. [Google Scholar] [CrossRef]
- Yu, R.; Tan, Y.; Yao, H.; Xu, Y.; Huang, J.; Zhao, B.; Du, Y.; Hua, Z.; Li, J.; Shi, J. Toward n-Alkane Hydroisomerization Reactions: High-Performance Pt-Al2O3/SAPO-11 Single-Atom Catalysts with Nanoscale Separated Metal-Acid Centers and Ultralow Platinum Content. ACS Appl. Mater. Interfaces 2022, 14, 44377–44388. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Shi, F.; Zhou, W.; Yang, Z.; Zhou, A. Hydroisomerization of 1-octene utilizing hierarchical SAPO-11-supported Ni catalysts: Effect of the alkyl chain length of the mesoporogen. New J. Chem. 2022, 46, 3901–3908. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Zhu, P.; Liu, H.; Zhang, X. Mercaptosilane-assisted synthesis of highly dispersed and stable Pt nanoparticles on HL zeolites for enhancing hydroisomerization of n-hexane. New J. Chem. 2022, 46, 3482–3492. [Google Scholar] [CrossRef]
- Guo, K.; Ma, A.; Wang, Z.; Li, J.; Wu, B.; Liu, T.; Li, D. Investigation of n-heptane hydroisomerization over alkali-acid-treated hierarchical Pt/ZSM-22 zeolites. New J. Chem. 2022, 46, 16752–16763. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Bibi, A.; Naqvi, M.; Noor, T.; Nizami, A.-S.; Rehan, M.; Ayoub, M. New trends in improving gasoline quality and octane through naphtha isomerization: A short review. Appl. Petrochem. Res. 2018, 8, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Fedyna, M.; Śliwa, M.; Jaroszewska, K.; Trawczyński, J. Effect of zeolite amount on the properties of Pt/(AlSBA-15 + Beta zeolite) micro-mesoporous catalysts for the hydroisomerization of n-heptane. Fuel 2020, 280, 118607. [Google Scholar] [CrossRef]
- Ali, N.S.; Alismaeel, Z.T.; Majdi, H.S.; Salih, H.G.; Abdulrahman, M.A.; Cata Saady, N.M.; Albayati, T.M. Modification of SBA-15 mesoporous silica as an active heterogeneous catalyst for the hydroisomerization and hydrocracking of n-heptane. Heliyon 2022, 8, e09737. [Google Scholar] [CrossRef]
- Demikhova, N.R.; Rubtsova, M.I.; Kireev, G.A.; Cherednichenko, K.A.; Vinokurov, V.A.; Glotov, A.P. Micro-mesoporous catalysts based on ZSM-5 zeolite synthesized from natural clay nanotubes: Preparation and application in the isomerization of C-8 aromatic fraction. Chem. Eng. J. 2023, 453, 139581. [Google Scholar] [CrossRef]
- Kapteijn, F.; Wang, X. Zeolite Membranes—The Importance of Support Analysis. Chem. Ing. Tech. 2022, 94, 23–30. [Google Scholar] [CrossRef]
- Gomez, L.Q.; Shehab, A.K.; Al-Shathr, A.; Ingram, W.; Konstantinova, M.; Cumming, D.; McGregor, J. H2-free Synthesis of Aromatic, Cyclic and Linear Oxygenates from CO2. ChemSusChem 2020, 13, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Wang, Z.; Chen, T.; Hu, L.; Yang, S.; Kawi, S. State-of-art designs and synthesis of zeolite membranes for CO2 capture. Carbon Capture Sci. Technol. 2022, 5, 100073. [Google Scholar] [CrossRef]
- Khadim, A.T.; Albayati, T.M.; Saady, N.M.C. Removal of sulfur compounds from real diesel fuel employing the encapsulated mesoporous material adsorbent Co/MCM-41 in a fixed-bed column. Microporous Mesoporous Mater. 2022, 341, 112020. [Google Scholar] [CrossRef]
- Ali, N.S.; Harharah, H.N.; Salih, I.K.; Saady, N.M.C.; Zendehboudi, S. Applying MCM-48 mesoporous material, equilibrium, isotherm, and mechanism for the effective adsorption of 4-nitroaniline from wastewater. Sci. Rep. 2023, 13, 9837. [Google Scholar] [CrossRef]
- Wenten, I.G.; Khoiruddin, K.; Mukti, R.R.; Rahmah, W.; Wang, Z.; Kawi, S. Zeolite membrane reactors: From preparation to application in heterogeneous catalytic reactions. React. Chem. Eng. 2021, 6, 401–417. [Google Scholar] [CrossRef]
- Alismaeel, Z.T.; Al-Jadir, T.M.; Albayati, T.M.; Abbas, A.S.; Doyle, A.M. Modification of FAU zeolite as an active heterogeneous catalyst for biodiesel production and theoretical considerations for kinetic modeling. Adv. Powder Technol. 2022, 33, 103646. [Google Scholar] [CrossRef]
- Dragomirova, R.; Wohlrab, S. Zeolite Membranes in Catalysis—From Separate Units to Particle Coatings. Catalysts 2015, 5, 2161–2222. [Google Scholar] [CrossRef]
- Ban, Y.; Yang, W. Multidimensional Building Blocks for Molecular Sieve Membranes. Acc. Chem. Res. 2022, 55, 3162–3177. [Google Scholar] [CrossRef]
- Shi, P.; Li, Z.; Chen, X.; Zeng, L.; Hu, R. Effect of industrial waste molecular sieves on internally cured cement-based materials. Front. Mater. 2022, 9, 1003556. [Google Scholar] [CrossRef]
- Mittal, N.; Bai, P.; Kelloway, A.; Siepmann, J.I.; Daoutidis, P.; Tsapatsis, M. A mathematical model for zeolite membrane module performance and its use for techno-economic evaluation of improved energy efficiency hybrid membrane-distillation processes for butane isomer separations. J. Membr. Sci. 2016, 520, 434–449. [Google Scholar] [CrossRef] [Green Version]
- Usman, M. Recent Progress of SAPO-34 Zeolite Membranes for CO2 Separation: A Review. Membranes 2022, 12, 507. [Google Scholar] [CrossRef]
- Gora, L.; Jansen, J.C. Hydroisomerization of C6 with a zeolite membrane reactor. J. Catal. 2005, 230, 269–281. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Jiang, T.; Zhang, J.; Zhang, C.; Sun, C.; Deng, Y.; Sun, J.; Wang, S. The investigation of MCM-48-type and MCM-41-type mesoporous silica as oral solid dispersion carriers for water insoluble cilostazol. Drug Dev. Ind. Pharm. 2014, 40, 819–828. [Google Scholar] [CrossRef]
- Kim, T.-W.; Kleitz, F.; Paul, B.; Ryoo, R. MCM-48-like Large Mesoporous Silicas with Tailored Pore Structure: Facile Synthesis Domain in a Ternary Triblock Copolymer−Butanol−Water System. J. Am. Chem. Soc. 2005, 127, 7601–7610. [Google Scholar] [CrossRef]
- Olivieri, F.; Castaldo, R.; Cocca, M.; Gentile, G.; Lavorgna, M. Mesoporous silica nanoparticles as carriers of active agents for smart anticorrosive organic coatings: A critical review. Nanoscale 2021, 13, 9091–9111. [Google Scholar] [CrossRef]
- Yu, Q.; Zhuang, R.; Yi, H.; Gao, W.; Zhang, Y.; Tang, X. Application of MCM-48 with large specific surface area for VOCs elimination: Synthesis and hydrophobic functionalization for highly efficient adsorption. Environ. Sci. Pollut. Res. 2022, 29, 33595–33608. [Google Scholar] [CrossRef]
- Li, M.; Dong, L.Y.; Li, X.X.; Guo, Z.P.; He, Y.; Lin, Q. Synthesis and Characterization of MCM-48 Molecular Sieves with High Specific Surface Area. Mater. Sci. Forum 2021, 1018, 147–152. [Google Scholar] [CrossRef]
- Albayati, T.M.; Doyle, A.M. SBA-15 Supported Bimetallic Catalysts for Enhancement Isomers Production During n-Heptane Decomposition. Int. J. Chem. React. Eng. 2014, 12, 345–354. [Google Scholar] [CrossRef]
- Albayati, T.M.; Wilkinson, S.E.; Garforth, A.A. Heterogeneous Alkane Reactions over Nanoporous Catalysts. Transp. Porous Media 2014, 104, 315–333. [Google Scholar] [CrossRef]
- Al-Nayili, A.; Majdi, H.S.; Albayati, T.M.; Saady, N.M.C. Formic Acid Dehydrogenation Using Noble-Metal Nanoheterogeneous Catalysts: Towards Sustainable Hydrogen-Based Energy. Catalysts 2022, 12, 324. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Refay, N.M.; El-Sherbeeny, A.M.; El-Meligy, M.A. Insight into the Loading and Release Properties of MCM-48/Biopolymer Composites as Carriers for 5-Fluorouracil: Equilibrium Modeling and Pharmacokinetic Studies. ACS Omega 2020, 5, 11745–11755. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Zhang, Z.; Ouyang, G.; Hao, Z. Effects of mesoporous silica particle size and pore structure on the performance of polymer-mesoporous silica mixed matrix membranes. RSC Adv. 2021, 11, 36577–36586. [Google Scholar] [CrossRef]
- Petrisor, G.; Ficai, D.; Motelica, L.; Trusca, R.D.; Bîrcă, A.C.; Vasile, B.S.; Voicu, G.; Oprea, O.C.; Semenescu, A.; Ficai, A.; et al. Mesoporous Silica Materials Loaded with Gallic Acid with Antimicrobial Potential. Nanomaterials 2022, 12, 1648. [Google Scholar] [CrossRef]
- Zhang, K.; Yuan, E.-H.; Xu, L.L.; Xue, Q.-S.; Luo, C.; Albela, B.; Bonneviot, L. Preparation of High-Quality MCM-48 Mesoporous Silica and the Mode of Action of the Template. Eur. J. Inorg. Chem. 2012, 2012, 4183–4189. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Ficai, D.; Trusca, R.D.; Surdu, V.-A.; Voicu, G.; Oprea, O.C.; Ficai, A.; Andronescu, E. New Mesoporous Silica Materials Loaded with Polyphenols: Caffeic Acid, Ferulic Acid and p-Coumaric Acid as Dietary Supplements for Oral Administration. Materials 2022, 15, 7982. [Google Scholar] [CrossRef]
- Fathy, M.; Selim, H.; Shahawy, A.E.L. Chitosan/MCM-48 nanocomposite as a potential adsorbent for removing phenol from aqueous solution. RSC Adv. 2020, 10, 23417–23430. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation. Int. J. Electrochem. Sci. 2012, 7, 4871–4888. [Google Scholar] [CrossRef]
- Eswaramoorthi, I.; Lingappan, N. Ni–Pt/H-Y Zeolite Catalysts for Hydroisomerization of n-Hexane and n-Heptane. Catal. Lett. 2003, 87, 133–142. [Google Scholar] [CrossRef]
- Al-Shathr, A.; Al-Zaidi, B.Y.; Shehab, A.K.; Shakoor, Z.M.; Aal-Kaeb, S.; Gomez, L.Q.; Majdi, H.S.; Al-Shafei, E.N.; AbdulRazak, A.A.; McGregor, J. Experimental and kinetic studies of the advantages of coke accumulation over Beta and Mordenite catalysts according to the pore mouth catalysis hypothesis. Catal. Commun. 2023, 181, 106718. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, R.; Cheng, J.; Zhao, H.; Du, Y.; Yao, H.; Li, J. Sinter-resistant platinum nanocatalysts immobilized by biochar for alkane hydroisomerization. Catal. Sci. Technol. 2021, 11, 7740–7750. [Google Scholar] [CrossRef]
- Chica, A.; Corma, A. Hydroisomerization of Pentane, Hexane, and Heptane for Improving the Octane Number of Gasoline. J. Catal. 1999, 187, 167–176. [Google Scholar] [CrossRef]
- Al-Shathr, A.; Shakor, Z.M.; Al-Zaidi, B.Y.; Majdi, H.S.; AbdulRazak, A.A.; Aal-Kaeb, S.; Shohib, A.A.; McGregor, J. Reaction Kinetics of Cinnamaldehyde Hydrogenation over Pt/SiO2: Comparison between Bulk and Intrapartcle Diffusion Models. Int. J. Chem. Eng. 2022, 2022, 8303874. [Google Scholar] [CrossRef]
- Wang, Z.; Navarrete, J. Keggin structure and surface acidity of 12-phospho-tungstic acid grafted Zr-MCM-48 mesoporous molecular sieves. World J. Nano Sci. Eng. 2012, 2, 134. [Google Scholar] [CrossRef]
- Toosi, M.R.; Peyrovi, M.H.; Mondgarian, R. Conversion of n-heptane to LPG and aromatics over Mo2C and W2C catalysts supported on ZSM-5. React. Kinet. Catal. Lett. 2009, 98, 133–138. [Google Scholar] [CrossRef]
- Zakumbaeva, G.; Van, T.; Lyashenko, A.; Egizbaeva, R. Catalytic reforming n-octane on Pt–Re/Al2O3 catalysts promoted by different additives. Catal. Today 2001, 65, 191–194. [Google Scholar] [CrossRef]
- Van Deelen, T.W.; Harmel, J.M.; Nijhuis, J.J.; Su, H.; Yoshida, H.; Oord, R.; Zečević, J.; Weckhuysen, B.M.; De Jong, K.P. Disk-Shaped Cobalt Nanocrystals as Fischer–Tropsch Synthesis Catalysts Under Industrially Relevant Conditions. Top. Catal. 2020, 63, 1398–1411. [Google Scholar] [CrossRef]
- Holger, L.; Hendrik, K.; Uwe, K.; Rolf, F. Acidity of substituted mesoporous molecular sieve MCM-48. J. Chem. Soc. Faraday Trans. 1998, 94, 971–977. [Google Scholar] [CrossRef]
- Doyle, A.M.; Ahmed, E.; Hodnett, B.K. The evolution of phases during the synthesis of the organically modified catalyst support MCM-48. Catal. Today 2006, 116, 50–55. [Google Scholar] [CrossRef]
- Doyle, A.; Hodnett, B.K. Synthesis of 2-cyanoethyl-modified MCM-48 stable to surfactant removal by solvent extraction: Influence of organic modifier, base and surfactant. Microporous Mesoporous Mater. 2003, 58, 255–261. [Google Scholar] [CrossRef]
- Hägg, M.B.; Lie, J.A.; Lindbråthen, A. Carbon molecular sieve membranes: A promising alternative for selected industrial applications. Ann. N. Y. Acad. Sci. 2003, 984, 329–345. [Google Scholar] [CrossRef]








| Sample | SBET (m2·g−1) | VP (cm3·g−1) | VµP (cm3·g−1) | DP (nm) | ɑₒ (nm) | twall (nm) |
|---|---|---|---|---|---|---|
| MCM-48 | 1400 | 1.3 | 0.4 | 3 | 4 | 0.5 |
| 3 wt.% Co/MCM-48 | 800 | 0.8 | 0.05 | 4 | 3 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, N.S.; Salih, I.K.; Harharah, H.N.; Majdi, H.S.; Salih, H.G.; Kalash, K.R.; Al-Shathr, A.; Al-Sudani, F.T.; Abdulrahman, M.A.; Alrubaye, J.M.; et al. Utilization of Loaded Cobalt onto MCM-48 Mesoporous Catalyst as a Heterogeneous Reaction in a Fixed Bed Membrane Reactor to Produce Isomerization Product from n-Heptane. Catalysts 2023, 13, 1138. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13071138
Ali NS, Salih IK, Harharah HN, Majdi HS, Salih HG, Kalash KR, Al-Shathr A, Al-Sudani FT, Abdulrahman MA, Alrubaye JM, et al. Utilization of Loaded Cobalt onto MCM-48 Mesoporous Catalyst as a Heterogeneous Reaction in a Fixed Bed Membrane Reactor to Produce Isomerization Product from n-Heptane. Catalysts. 2023; 13(7):1138. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13071138
Chicago/Turabian StyleAli, Nisreen S., Issam K. Salih, Hamed N. Harharah, Hasan Sh. Majdi, Hussein G. Salih, Khairi R. Kalash, Ali Al-Shathr, Farah T. Al-Sudani, Mahir A. Abdulrahman, Jamal M. Alrubaye, and et al. 2023. "Utilization of Loaded Cobalt onto MCM-48 Mesoporous Catalyst as a Heterogeneous Reaction in a Fixed Bed Membrane Reactor to Produce Isomerization Product from n-Heptane" Catalysts 13, no. 7: 1138. https://0-doi-org.brum.beds.ac.uk/10.3390/catal13071138