Ru/Attapulgite as an Efficient and Low-Cost Ammonia Decomposition Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure and Elementary Composition Analysis of ATP
2.2. Thermal Stability and Morphology of Natural ATP
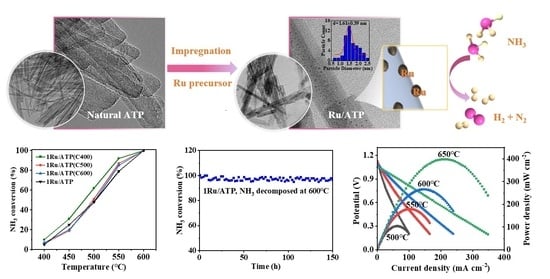
2.3. Characterization and Evaluation of the Ru-ATP Catalyst
2.4. NH3-Fed Proton Ceramic Fuel Cells with 1Ru/ATP
3. Materials and Methods
3.1. Preparation of the Samples
3.2. Characterization of the Samples
3.3. Catalytic Performance Test
3.4. Fabrication and Electrochemical Characterization of Fuel Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, R.; Kawanami, H. A Recent Review of Primary Hydrogen Carriers, Hydrogen Production Methods, and Applications. Catalysts 2023, 13, 562. [Google Scholar] [CrossRef]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-fed fuel cells: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- Sun, S.C.; Jiang, Q.Q.; Zhao, D.Y.; Cao, T.T.; Sha, H.; Zhang, C.K.; Song, H.T.; Da, Z. Ammonia as hydrogen carrier: Advances in ammonia decomposition catalysts for promising hydrogen production. Renew. Sustain. Energy Rev. 2022, 169, 112918. [Google Scholar] [CrossRef]
- Wang, B.; Li, T.; Gong, F.; Othman, M.H.D.; Xiao, R. Ammonia as a green energy carrier: Electrochemical synthesis and direct ammonia fuel cell-a comprehensive review. Fuel Process. Technol. 2022, 235, 107380. [Google Scholar] [CrossRef]
- Yi, Y.; Chen, J.; Xu, M.; Yang, G.; Ran, R.; Zhou, W.; Wang, W.; Shao, Z. Exsolved Nanoparticles Decorated Double Perovskites as High-Performance Anodes for Direct-Ammonia Solid Oxide Fuel Cells. Catalysts 2023, 13, 996. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S. Recent advances in ammonia synthesis technologies: Toward future zero carbon emissions. Int. J. Hydrogen Energy 2023, 48, 11237–11273. [Google Scholar] [CrossRef]
- Zhu, L.Z.; Cadigan, C.; Duan, C.C.; Huang, J.K.; Bian, L.Z.; Le, L.; Hernandez, C.H.; Avance, V.; O’Hayre, R.; Sullivan, N.P. Ammonia-fed reversible protonic ceramic fuel cells with Ru-based catalyst. Commun. Chem. 2021, 4, 121. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.A.; Iost, K.N.; Temerev, V.L.; Simunin, M.M.; Leont’eva, N.N.; Mikhlin, Y.L.; Volochaev, M.N.; Shlyapin, D.A. Ammonia decomposition Ru catalysts supported on alumina nanofibers for hydrogen generation. Mater. Lett. 2022, 306, 130842. [Google Scholar] [CrossRef]
- Duan, X.Z.; Qian, G.; Zhou, X.G.; Sui, Z.J.; Chen, D.; Yuan, W.K. Tuning the size and shape of Fe nanoparticles on carbon nanofibers for catalytic ammonia decomposition. Appl. Catal. B-Environ. 2011, 101, 189–196. [Google Scholar] [CrossRef]
- Atsumi, R.; Noda, R.; Takagi, H.; Vecchione, L.; Di Carlo, A.; Del Prete, Z.; Kuramoto, K. Ammonia decomposition activity over Ni/SiO2 catalysts with different pore diameters. Int. J. Hydrogen Energy 2014, 39, 13954–13961. [Google Scholar] [CrossRef]
- Papapolymerou, G.; Bontozoglou, V. Decomposition of NH3 on Pd and Ir—Comparison with Pt and Rh. J. Mol. Catal. A-Chem. 1997, 120, 165–171. [Google Scholar] [CrossRef]
- Yakovenko, R.E.; Krasnyakova, T.V.; Saliev, A.N.; Shilov, M.A.; Volik, A.V.; Savost’yanov, A.P.; Mitchenko, S.A. Ammonia Decomposition over Cobalt-Based Silica-Supported Fischer-Tropsch Synthesis Catalysts. Kinet. Catal. 2023, 64, 180–190. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Y.; Guo, H.; Du, X.; Zhu, B.; Zhu, Y. Highly Dispersed Co Nanoparticles Prepared by an Improved Method for Plasma-Driven NH3 Decomposition to Produce H2. Catalysts 2019, 9, 107. [Google Scholar] [CrossRef]
- Lucentini, I.; Colli, G.G.; Luzi, C.D.; Serrano, I.; Martínez, O.M.; Llorca, J. Catalytic ammonia decomposition over Ni-Ru supported on CeO2 for hydrogen production: Effect of metal loading and kinetic analysis. Appl. Catal. B-Environ. 2021, 286, 119896. [Google Scholar] [CrossRef]
- Lucentini, I.; Casanovas, A.; Llorca, J. Catalytic ammonia decomposition for hydrogen production on Ni, Ru and Ni-Ru supported on CeO2. Int. J. Hydrogen Energy 2019, 44, 12693–12707. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.W.; Ali, A.M.; Luo, W.J.; Wen, J.; Zhang, L.H.; Zhang, H. Bimetallic Ru-Fe Nanoparticles Supported on Carbon Nanotubes for Ammonia Decomposition and Synthesis. Chem. Eng. Technol. 2020, 43, 719–730. [Google Scholar] [CrossRef]
- Wu, Z.W.; Li, X.; Qin, Y.H.; Deng, L.D.; Wang, C.W.; Jiang, X.M. Ammonia decomposition over SiO2-supported Ni-Co bimetallic catalyst for COx-free hydrogen generation. Int. J. Hydrogen Energy 2020, 45, 15263–15269. [Google Scholar] [CrossRef]
- Zhang, J.; Müller, J.O.; Zheng, W.Q.; Wang, D.; Su, D.S.; Schlögl, R. Individual Fe-Co alloy nanoparticles on carbon nanotubes: Structural and catalytic properties. Nano Lett. 2008, 8, 2738–2743. [Google Scholar] [CrossRef]
- Duan, X.Z.; Ji, J.; Yan, X.D.; Qian, G.; Chen, D.; Zhou, X.G. Understanding Co-Mo Catalyzed Ammonia Decomposition: Influence of Calcination Atmosphere and Identification of Active Phase. Chemcatchem 2016, 8, 938–945. [Google Scholar] [CrossRef]
- Yi, Y.H.; Wang, L.; Guo, Y.J.; Sun, S.Q.; Guo, H.C. Plasma-assisted ammonia decomposition over Fe-Ni alloy catalysts for COx-Free hydrogen. Aiche J. 2019, 65, 691–701. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhou, X.P.; Au, C.T. A mini-review on ammonia decomposition catalysts for on-site generation of hydrogen for fuel cell applications. Appl. Catal. A-Gen. 2004, 277, 1–9. [Google Scholar] [CrossRef]
- Duan, X.Z.; Zhou, J.H.; Qian, G.; Li, P.; Zhou, X.G.; Chen, D. Carbon Nanofiber-Supported Ru Catalysts for Hydrogen Evolution by Ammonia Decomposition. Chin. J. Catal. 2010, 31, 979–986. [Google Scholar] [CrossRef]
- Karakaya, C.; Huang, J.; Cadigan, C.; Welch, A.; Kintner, J.; Beach, J.; Zhu, H.Y.; O’Hayre, R.; Kee, R.J. Development, characterization, and modeling of a high-performance Ru/B2CA catalyst for ammonia synthesis. Chem. Eng. Sci. 2022, 247, 116902. [Google Scholar] [CrossRef]
- Li, G.; Kanezashi, M.; Tsuru, T. Catalytic Ammonia Decomposition over High-Performance Ru/Graphene Nanocomposites for Efficient COx-Free Hydrogen Production. Catalysts 2017, 7, 23. [Google Scholar] [CrossRef]
- Fang, H.H.; Wu, S.; Ayvali, T.; Zheng, J.; Fellowes, J.; Ho, P.L.; Leung, K.C.; Large, A.; Held, G.; Kato, R.; et al. Dispersed surface Ru ensembles on MgO(111) for catalytic ammonia decomposition. Nat. Commun. 2023, 14, 647. [Google Scholar] [CrossRef]
- Hara, M.; Kitano, M.; Hosono, H. Ru-Loaded C12A7:e- Electride as a Catalyst for Ammonia Synthesis. ACS Catal. 2017, 7, 2313–2324. [Google Scholar] [CrossRef]
- Li, G.R.; Tan, Y.H.; Lei, Z.P.; Yin, F.X.; He, X.B. Ni Nanoparticles Supported on High Surface Area Carborundum for Enhanced Hydrogen Production by Ammonia Decomposition. Acs. Appl. Nano Mater. 2023, 6, 9892–9900. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, S.; Xiong, X.; Hu, B.; Song, C. Effect of Graphitic Carbon Nitride on the Electronic and Catalytic Properties of Ru Nanoparticles for Ammonia Synthesis. Catal. Lett. 2016, 146, 2324–2329. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Juang, C.-F.; Chen, Y.-C. The Effects of Promoter Cs Loading on the Hydrogen Production from Ammonia Decomposition Using Ru/C Catalyst in a Fixed-Bed Reactor. Catalysts 2021, 11, 321. [Google Scholar] [CrossRef]
- Baek, S.-H.; Yun, K.; Kang, D.-C.; An, H.; Park, M.B.; Shin, C.-H.; Min, H.-K. Characteristics of High Surface Area Molybdenum Nitride and Its Activity for the Catalytic Decomposition of Ammonia. Catalysts 2021, 11, 192. [Google Scholar] [CrossRef]
- Hayashi, F.; Toda, Y.; Kanie, Y.; Kitano, M.; Inoue, Y.; Yokoyama, T.; Hara, M.; Hosono, H. Ammonia decomposition by ruthenium nanoparticles loaded on inorganic electride C12A7:e−. Chem. Sci. 2013, 4, 3124–3130. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, H.; Xu, K.; Wang, W.; Jia, C. CeO2−x modified Ru/γ-Al2O3 catalysts for ammonia decomposition reaction. J. Rare Earths 2023, 41, 801–809. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, E.D. Ammonia Decomposition over Ru/SiO2 Catalysts. Catalysts 2022, 12, 1203. [Google Scholar] [CrossRef]
- Yin, S.F.; Zhang, Q.H.; Xu, B.Q.; Zhu, W.X.; Ng, C.F.; Au, C.T. Investigation on the catalysis of COx-free hydrogen generation from ammonia. J. Catal. 2004, 224, 384–396. [Google Scholar] [CrossRef]
- Podila, S.; Al-Zahrani, A.A.; Pasupulety, N.; Alamoudi, M.A. Influence of CaCe ratio on the hydrogen production from ammonia over CaO-CeO2 supported Co catalysts. Arab. J. Chem. 2023, 16, 105235. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Qu, Y.M.; Shen, X.L.; Cai, Z.F. Ruthenium catalyst supported on Ba modified ZrO2 for ammonia decomposition to COx-free hydrogen. Int. J. Hydrogen Energy 2019, 44, 7300–7307. [Google Scholar] [CrossRef]
- Tan, H.; Li, K.; Sioud, S.; Cha, D.; Amad, M.H.; Hedhili, M.N.; Al-Talla, Z.A. Synthesis of Ru nanoparticles confined in magnesium oxide-modified mesoporous alumina and their enhanced catalytic performance during ammonia decomposition. Catal. Commun. 2012, 26, 248–252. [Google Scholar] [CrossRef]
- Pachoulis, M.; Sapalidis, A.A.; Kouvelos, E.P.; Gotzias, A.; Kyzas, G.Z.; Favvas, E.P. Study of Cu2+ and dyes removal by sorption onto palygorskite in batch and continuous flow processes. Desalination Water Treat. 2022, 255, 101–109. [Google Scholar] [CrossRef]
- Lokanatha, S.; Mathur, B.K.; Samantaray, B.K.; Bhattacherjee, S. Dehydration and phase-transformation in attapulgite (palygorskite)-an R.D.F. study. J. Mater. Sci. Lett. 1984, 3, 1105–1108. [Google Scholar] [CrossRef]
- Wang, Y.S.; Chen, M.Q.; Yang, Z.L.; Liang, T.; Liu, S.M.; Zhou, Z.S.; Li, X.J. Bimetallic Ni-M (M = Co, Cu and Zn) supported on attapulgite as catalysts for hydrogen production from glycerol steam reforming. Appl. Catal. A-Gen. 2018, 550, 214–227. [Google Scholar] [CrossRef]
- Suárez, M.; García-Romero, E. FTIR spectroscopic study of palygorskite: Influence of the composition of the octahedral sheet. Appl. Clay Sci. 2006, 31, 154–163. [Google Scholar] [CrossRef]
- Suárez, M.; García-Romero, E. Chapter 2—Advances in the Crystal Chemistry of Sepiolite and Palygorskite. In Developments in Clay Science; Galàn, E., Singer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 3, pp. 33–65. [Google Scholar]
- Bradley, W.F. The structural scheme of attapulgite. Am. Mineral. 1940, 25, 405–410. [Google Scholar]
- Galan, E.; Carretero, M.I. A new approach to compositional limits for sepiolite and palygorskite. Clays Clay Miner. 1999, 47, 399–409. [Google Scholar] [CrossRef]
- Post, J.E.; Heaney, P.J. Synchrotron powder X-ray diffraction study of the structure and dehydration behavior of palygorskite. Am. Mineral. 2008, 93, 667–675. [Google Scholar] [CrossRef]
- Lu, Y.S.; Wang, A.Q. From structure evolution of palygorskite to functional material: A review. Microporous Mesoporous Mater. 2022, 333, 111765. [Google Scholar] [CrossRef]
- Suárez, M.; Romero, E.A. Macroscopic palygorskite from Lisbom Volcanic Complex. Eur. J. Miner. 2006, 18, 119–126. [Google Scholar] [CrossRef]
- Ogorodova, L.; Vigasina, M.; Melchakova, L.; Krupskaya, V.; Kiseleva, I. Thermochemical study of natural magnesium aluminum phyllosilicate: Palygorskite. J. Chem. Thermodyn. 2015, 89, 205–211. [Google Scholar] [CrossRef]
- Huang, Y.J.; Li, Z.; Li, S.Z.; Shi, Z.L.; Yin, L.; Hsia, Y.F. Mossbauer investigations of palygorskite from Xuyi, China. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 2007, 260, 657–662. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, J. 13—Maya Blue Pigments Derived From Clay Minerals. In Nanomaterials from Clay Minerals; Wang, A., Wang, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 627–661. [Google Scholar]
- Yang, Q.L.; Wu, H.L.; Zhan, H.Y.; Hou, J.J.; Gao, M.E.; Su, Q.; Wu, S. Attapulgite-anchored Pd complex catalyst: A highly active and reusable catalyst for C-C coupling reactions. React. Kinet. Mech. Catal. 2020, 129, 283–295. [Google Scholar] [CrossRef]
- Chen, M.Q.; Zhou, Z.S.; Wang, Y.S.; Liang, T.; Li, X.J.; Yang, Z.L.; Chen, M.G.; Wang, J. Effects of attapulgite-supported transition metals catalysts on glycerol steam reforming for hydrogen production. Int. J. Hydrogen Energy 2018, 43, 20451–20464. [Google Scholar] [CrossRef]
- Feng, P.; Huang, K.; Xu, Q.; Qi, W.; Xin, S.; Wei, T.; Liao, L.; Yan, Y. Ni supported on the CaO modified attapulgite as catalysts for hydrogen production from glycerol steam reforming. Int. J. Hydrogen Energy 2020, 45, 8223–8233. [Google Scholar] [CrossRef]
- Xu, Q.L.; Zhang, Z.D.; Huang, K.; Xin, S.Z.; Mao, C.; Chen, C.G.; Yang, H.; Yue, Y.; Yan, Y.J. Ni supported on MgO modified attapulgite as catalysts for hydrogen production from glycerol steam reforming. Int. J. Hydrogen Energy 2021, 46, 27380–27393. [Google Scholar] [CrossRef]
- Cao, J.L.; Shao, G.S.; Wang, Y.; Liu, Y.P.; Yuan, Z.Y. CuO catalysts supported on attapulgite clay for low-temperature CO oxidation. Catal. Commun. 2008, 9, 2555–2559. [Google Scholar] [CrossRef]
- Cao, L.; Lu, M.H.; Li, G.; Zhang, S.Y. Hydrogen production from methanol steam reforming catalyzed by Fe modified Cu supported on attapulgite clay. React. Kinet. Mech. Catal. 2019, 126, 137–152. [Google Scholar] [CrossRef]
- Chen, M.Q.; Sun, G.W.; Wang, Y.S.; Liang, D.F.; Li, C.; Wang, J.; Liu, Q. Steam reforming of methanol for hydrogen production over attapulgite-based zeolite-supported Cu-Zr catalyst. Fuel 2022, 314, 122733. [Google Scholar] [CrossRef]
- Li, Y.X.; Yao, L.H.; Song, Y.Y.; Liu, S.Q.; Zhao, J.; Ji, W.J.; Au, C.T. Core-shell structured microcapsular-like Ru@SiO2 reactor for efficient generation of COx-free hydrogen through ammonia decomposition. Chem. Commun. 2010, 46, 5298–5300. [Google Scholar] [CrossRef]
- Christ, C.L.; Hathaway, J.C.; Hostetle, P.B.; Shepard, A.O. Palygorskite: New X-ray data. Am. Mineral. 1969, 54, 198–205. [Google Scholar]
- Chisholm, J.E. Powder-Diffraction Patterns and Structural Models for Palygorskite. Can. Mineral. 1992, 30, 61–73. [Google Scholar]
- Wang, Y.S.; Liang, D.F.; Wang, C.S.; Chen, M.Q.; Tang, Z.Y.; Hu, J.X.; Yang, Z.L.; Zhang, H.; Wang, J.; Liu, S.M. Influence of calcination temperature of Ni/Attapulgite on hydrogen production by steam reforming ethanol. Renew. Energy 2020, 160, 597–611. [Google Scholar] [CrossRef]
- Suárez, M.; García-Romer, E.; del Rio, M.S.; Martinetto, P.; Dooryhée, E. The effect of the octahedral cations on the dimensions of the palygorskite cell. Clay Miner. 2007, 42, 287–297. [Google Scholar] [CrossRef]
- García-Romero, E.; Suárez, M. On the Chemical Composition of Sepiolite and Palygorskite. Clays Clay Miner. 2010, 58, 1–20. [Google Scholar] [CrossRef]
- Guo, R.X.; Wang, G.; Liu, W.S. Clever use of natural clay materials in the synthesis of non-symmetric carbonates by utilizing CO2 as a feedstock: Ag/attapulgite nano-catalyst. Dalton Trans. 2020, 49, 10232–10239. [Google Scholar] [CrossRef]
- Li, L.; Chen, F.; Shao, J.L.; Dai, Y.; Ding, J.F.; Tang, Z. Attapulgite clay supported Ni nanoparticles encapsulated by porous silica: Thermally stable catalysts for ammonia decomposition to COx free hydrogen. Int. J. Hydrogen Energy 2016, 41, 21157–21165. [Google Scholar] [CrossRef]
- Mo, X.X.; Zhuang, Z.Y.; Ren, C.; Li, W. Thermal activation of palygorskite for enhanced fluoride removal under alkaline conditions. Appl. Geochem. 2022, 147, 105484. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Ng, C.F.; Au, C.T. Nano Ru/CNTs: A highly active and stable catalyst for the generation of COx-free hydrogen in ammonia decomposition. Appl. Catal. B-Environ. 2004, 48, 237–241. [Google Scholar] [CrossRef]
- Ren, S.; Huang, F.; Zheng, J.; Chen, S.J.; Zhang, H. Ruthenium supported on nitrogen-doped ordered mesoporous carbon as highly active catalyst for NH3 decomposition to H2. Int. J. Hydrogen Energy 2017, 42, 5105–5113. [Google Scholar] [CrossRef]
- Zhao, J.W.; Xu, S.; Wu, H.J.; You, Z.X.; Deng, L.D.; Qiu, X.H. Metal-support interactions on Ru/CaAlOx catalysts derived from structural reconstruction of Ca-Al layered double hydroxides for ammonia decomposition. Chem. Commun. 2019, 55, 14410–14413. [Google Scholar] [CrossRef]
- Fang, H.H.; Liu, D.; Luo, Y.; Zhou, Y.L.; Liang, S.J.; Wang, X.Y.; Lin, B.Y.; Jiang, L.L. Challenges and Opportunities of Ru-Based Catalysts toward the Synthesis and Utilization of Ammonia. ACS Catal. 2022, 12, 3938–3954. [Google Scholar] [CrossRef]
- Li, G.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Graphene nanosheets supporting Ru nanoparticles with controlled nanoarchitectures form a high-performance catalyst for COx-free hydrogen production from ammonia. J. Mater. Chem. A 2014, 2, 9185–9192. [Google Scholar] [CrossRef]
- Chen, C.Q.; Wu, K.; Ren, H.J.; Zhou, C.; Luo, Y.; Lin, L.; Au, C.T.; Jiang, L.L. Ru-Based Catalysts for Ammonia Decomposition: A Mini-Review. Energy Fuels 2021, 35, 11693–11706. [Google Scholar] [CrossRef]
- Im, Y.; Muroyama, H.; Matsui, T.; Eguchi, K. Investigation on catalytic performance and desorption behaviors of ruthenium catalysts supported on rare-earth oxides for NH3 decomposition. Int. J. Hydrogen Energy 2022, 47, 32543–32551. [Google Scholar] [CrossRef]
- Hu, Z.G.; Mahin, J.; Datta, S.; Bell, T.E.; Torrente-Murciano, L. Ru-Based Catalysts for H2 Production from Ammonia: Effect of 1D Support. Top. Catal. 2019, 62, 1169–1177. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, Y.S.; Chen, M.Q.; Liang, D.F.; Li, C.; Yang, Z.L.; Wang, J. Hydrogen production from aqueous phase reforming of glycerol over attapulgite-supported nickel catalysts: Effect of acid/base treatment and Fe additive. Int. J. Hydrogen Energy 2022, 47, 7082–7099. [Google Scholar] [CrossRef]
- Krekeler, M.P.S.; Guggenheim, S. Defects in microstructure in palygorskite–sepiolite minerals: A transmission electron microscopy (TEM) study. Appl. Clay Sci. 2008, 39, 98–105. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Palygorskite Nanomaterials: Structure, Properties, and Functional Applications. In Nanomaterials from Clay Minerals; 2019; pp. 21–133. [Google Scholar] [CrossRef]
- Jing, J.M.; Lei, Z.; Wang, C.Y.; Zheng, Z.W.; Wang, H.R.; Zhang, P.P.; Yang, Z.B.; Peng, S.P. Boosting Performance of a Protonic Ceramic Fuel Cell by the Incorporation of Active Nano-Structured Layers. Acs Sustain. Chem. Eng. 2023, 11, 10303–10310. [Google Scholar] [CrossRef]
- Pan, Y.X.; Zhang, H.; Xu, K.; Zhou, Y.C.; Zhao, B.T.; Yuan, W.; Sasaki, K.; Choi, Y.; Chen, Y.; Liu, M.L. A high-performance and durable direct NH3 tubular protonic ceramic fuel cell integrated with an internal catalyst layer. Appl. Catal. B-Environ. Energy 2022, 306, 121071. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Y.S.; Yu, S.F.; Bi, L. A new CoFe1.9Li0.1O4 spinel oxide cathode for proton-conducting solid oxide fuel cells. Ceram. Int. 2022, 48, 34098–34104. [Google Scholar] [CrossRef]
- Xu, Y.S.; Xu, X.; Bi, L. A high-entropy spinel ceramic oxide as the cathode for proton-conducting solid oxide fuel cells. J. Adv. Ceram. 2022, 11, 794–804. [Google Scholar] [CrossRef]
- Chen, Y.; Yoo, S.; Pei, K.; Chen, D.C.; Zhang, L.; deGlee, B.; Murphy, R.; Zhao, B.T.; Zhang, Y.X.; Chen, Y.; et al. An In Situ Formed, Dual-Phase Cathode with a Highly Active Catalyst Coating for Protonic Ceramic Fuel Cells. Adv. Funct. Mater. 2018, 28, 1704907. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.C.; Pei, K.; Pan, Y.X.; Xu, K.; Ding, Y.; Zhao, B.T.; Sasaki, K.; Choi, Y.M.; Chen, Y.; et al. An efficient and durable anode for ammonia protonic ceramic fuel cells. Energy Environ. Sci. 2022, 15, 287–295. [Google Scholar] [CrossRef]
- Tao, H.; Ren, Q.; Zhang, Y.; Yang, L.; Teng, Q.; Xu, K.; Sang, J.; Guan, W.; Zhu, L. High-performance and stable proton ceramic fuel cells prepared via a co-tape casting process. Int. J. Hydrogen Energy 2024, 57, 1498–1505. [Google Scholar] [CrossRef]











| Xuyi, China T = 300 K | ICDD (31-0873) T = 300 K | Alaska *, USA T = 300 K | Alaska *, USA T = 1160 K | |
|---|---|---|---|---|
| System | Orthorhombic | Orthorhombic | Monoclinic | Monoclinic |
| a (Å) | 12.841 | 12.725 | 13.282 | 10.755 |
| b (Å) | 17.913 | 17.872 | 17.832 | 15.355 |
| c (Å) | 5.233 | 5.242 | 5.240 | 5.281 |
| β (o) | 90 | 90 | 107.66 | 96.17 |
| V (Å3) | 1203.7 | 1192.1 | 1182.5 | 867.1 |
| Elements | Si | IVAl 1 | VIAl 1 | Mg | Fe | Ti | Mn | (R2+/R3+) 2 | OC 3 | Ca | K | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molar ratio | 7.97 | 0.03 | 1.24 | 2.23 | 0.45 | 0.03 | 0.01 | 1.32 | 3.96 | 0.10 | 0.12 | 0.01 |
| Samples | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Diameter (nm) |
|---|---|---|---|
| ATP | 134.1 | 0.415 | 12.74 |
| ATP(C400) | 110.0 | 0.442 | 17.56 |
| ATP(C500) | 99.8 | 0.497 | 22.47 |
| ATP(C600) | 87.2 | 0.476 | 25.90 |
| Active Metals | Metal Content (wt%) | Support Materials | WHSV (mL g−1 h−1) | Temperature (°C) | Conversion (%) | Ref. |
|---|---|---|---|---|---|---|
| Ru | 5 | AC | 6000 | 450 | 9 | [68] |
| Ru | 3 | ZrO2 | 3000 | 500 | 43 | [36] |
| Ru | 3.5 | CNTs | 6000 | 450 | 21 | [69] |
| Ru | 2.5 | CaAlOx-e | 6000 | 500 | 85.5 | [69] |
| Ru | 2.5 | CaAlOx-w | 6000 | 500 | 98.2 | [69] |
| Ru3Fe | 1.98 | CNTs | 6000 | 500 | 100 | [16] |
| Ru | 2.03 | CNTs | 6000 | 500 | 100 | [16] |
| Ru | 2 | Y2O3 | 6000 | 500 | 82.6 | [73] |
| Ru | 2 | La2O3 | 6000 | 500 | 95.4 | [73] |
| Ru | 2 | PrxOy | 6000 | 500 | 93 | [73] |
| Ru | 2 | Sm2O3 | 6000 | 500 | 85.6 | [73] |
| Ru | 7 | Al2O3(nanorods) | 6000 | 500 | 100 | [74] |
| Ru | 7 | CNTs | 6000 | 500 | 93 | [74] |
| Ru | 1 | (BaO)2(CaO)(Al2O3) | 3000 | 500 | 100 | [7] |
| Ru | 1 | ATP | 6000 | 450 | 24 | This work |
| Ru | 1 | ATP | 6000 | 500 | 51 | This work |
| Ru | 1 | ATP | 6000 | 600 | 100 | This work |
| Temperatures (°C) | H2 | Cracked NH3 | ||
|---|---|---|---|---|
| Ro (Ω cm2) | Rp (Ω cm2) | Ro (Ω cm2) | Rp (Ω cm2) | |
| 500 | 0.75 | 2.26 | 0.69 | 3.33 |
| 550 | 0.56 | 0.93 | 0.49 | 1.43 |
| 600 | 0.43 | 0.43 | 0.37 | 0.67 |
| 650 | 0.35 | 0.20 | 0.30 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Q.; Sang, J.; Chen, G.; Tao, H.; Wang, Y.; Li, H.; Guan, W.; Ding, C.; Liu, F.; Zhu, L. Ru/Attapulgite as an Efficient and Low-Cost Ammonia Decomposition Catalyst. Catalysts 2024, 14, 197. https://0-doi-org.brum.beds.ac.uk/10.3390/catal14030197
Teng Q, Sang J, Chen G, Tao H, Wang Y, Li H, Guan W, Ding C, Liu F, Zhu L. Ru/Attapulgite as an Efficient and Low-Cost Ammonia Decomposition Catalyst. Catalysts. 2024; 14(3):197. https://0-doi-org.brum.beds.ac.uk/10.3390/catal14030197
Chicago/Turabian StyleTeng, Qingfeng, Junkang Sang, Guoxin Chen, Haoliang Tao, Yunan Wang, Hua Li, Wanbing Guan, Changsheng Ding, Fenghua Liu, and Liangzhu Zhu. 2024. "Ru/Attapulgite as an Efficient and Low-Cost Ammonia Decomposition Catalyst" Catalysts 14, no. 3: 197. https://0-doi-org.brum.beds.ac.uk/10.3390/catal14030197