Synergically Improving Light Harvesting and Charge Transportation of TiO2 Nanobelts by Deposition of MoS2 for Enhanced Photocatalytic Removal of Cr(VI)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterizations of MoS2/TiO2 NBs Heterojunction Composite
2.2. Photocatalytic Activity of MoS2/TiO2 NBs Heterojunctions
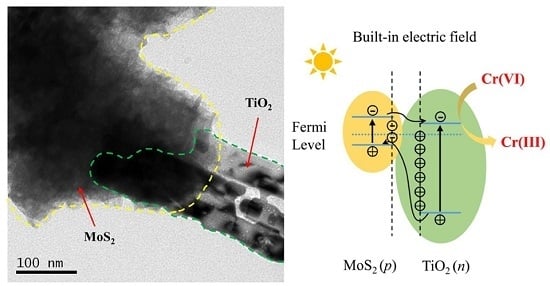
2.3. Photocatalytic Reduction Mechanism of MoS2/TiO2 NBs p-n Heterojunction
3. Experimental Section
3.1. Synthesis of TiO2 Nanobelts
3.2. Synthesis of MoS2/TiO2 NBs Heterojunction
3.3. Characterizations
3.4. Photocatalytic Activity Measurement
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, R.N.; Pan, X.H.; Li, F.; Zhang, L.; Zhai, S.M.; Mu, Q.X.; Liu, J.F.; Qu, G.B.; Jiang, G.B.; Yan, B. Cell rescue by nanosequestration: Reduced cytotoxicity of an environmental remediation residue, Mg(OH)2 nanoflake/Cr(VI) adduct. Environ. Sci. Technol. 2014, 48, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Bokare, A.D.; Choi, W. Advanced oxidation process based on the Cr(III)/Cr(VI) redox cycle. Environ. Sci. Technol. 2011, 45, 9332–9338. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Wang, S.L.; Lin, Y.C.; Wang, M.K.; Chiang, P.N.; Liu, J.C.; Kuan, W.H.; Chen, C.C.; Tzou, Y.M. Cr(VI) removal on fungal biomass of Neurospora crassa: The importance of dissolved organic carbons derived from the biomass to Cr(VI) reduction. Environ. Sci. Technol. 2010, 44, 6202–6208. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.Y.; Teng, W.; Zhao, Q.D.; Shi, Y.; Yue, R.L.; Chen, Y.F. Efficient photocatalytic reduction of aqueous Cr(VI) over flower-like SnIn4S8 microspheres under visible light illumination. J. Hazard. Mater. 2013, 244, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.P.; Lei, W.Y.; Liu, X.J.; Li, J.L.; Zheng, W.; Zhu, G.; Li, C.; Pan, L.K.; Sun, C.Q. Synergetic effect of TiO2 as co-catalyst for enhanced visible light photocatalytic reduction of Cr(VI) on MoSe2. Appl. Catal. A 2016, 521, 19–25. [Google Scholar] [CrossRef]
- Wang, H.G.; Wen, F.F.; Li, X.Y.; Gan, X.R.; Yang, Y.N.; Chen, P.; Zhang, Y. Cerium-doped MoS2 nanostructures: Efficient visible photocatalysis for Cr(VI) removal. Sep. Purif. Technol. 2016, 170, 190–198. [Google Scholar] [CrossRef]
- Yang, D.; Sun, Y.Y.; Tong, Z.W.; Nan, Y.H.; Jiang, Z.Y. Fabrication of bimodal-pore SrTiO3 microspheres with excellent photocatalytic performance for Cr(VI) reduction under simulated sunlight. J. Hazard. Mater. 2016, 312, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Baudys, M.; Krysa, J.; Zlamal, M.; Mills, A. Weathering tests of photocatalytic facade paints containing ZnO and TiO2. Chem. Eng. J. 2015, 261, 83–87. [Google Scholar] [CrossRef]
- Chen, H.H.; Nanayakkara, C.E.; Grassian, V.H. Titanium dioxide photocatalysis in atmospheric chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Bae, S.E.; Won, Y.S.; Huh, S. Simple preparation of lotus-root shaped meso-/macroporous TiO2 and their DSSC performances. J. Colloid Interface Sci. 2015, 448, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, D.W.; Li, C.; Xu, F.; Lei, W.; Sun, L.T.; Nathan, A.; Sun, X.W. All solution-processed stable white quantum dot light emitting diodes with hybrid ZnO@TiO2 as blue emitters. Sci. Rep. 2014, 4, 4085. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.J.; Yu, K.; Fu, H.; Hua, Q.Q.; Qi, R.J.; Li, H.L.; Song, H.L.; Guo, S.; Zhu, Z.Q. Firework-shaped TiO2 microspheres embedded with few-layer MoS2 as an anode material for excellent performance lithium-ion batteries. J. Mater. Chem. A 2015, 3, 6392–6401. [Google Scholar] [CrossRef]
- Tosoni, S.; Hevia, D.F.; Diaz, O.G.; Illas, F. Origin of optical excitations in fluorine-doped titania from response function theory: Relevance to photocatalysis. J. Phys. Chem. Lett. 2012, 3, 2269–2274. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.M.; Ming, L.F.; Chen, F. TiO2 with “fluorine-occupied” surface oxygen vacancies and its stably enhanced photocatalytic performance. ChemCatChem 2015, 7, 1797–1800. [Google Scholar] [CrossRef]
- Chen, Q.F.; Ma, W.H.; Chen, C.C.; Ji, H.W.; Zhao, J.C. Anatase TiO2 mesocrystals enclosed by (001) and (101) facets: Synergistic effects between Ti3+ and facets for their photocatalytic performance. Chem. Eur. J. 2012, 18, 12584–12589. [Google Scholar] [CrossRef] [PubMed]
- Ruoko, T.; Kaunisto, K.; Bartsch, M.; Pohjola, J.; Hiltunen, A.; Niederberger, M.; Tkachenko, N.V.; Lemmetyinen, H. Subpicosecond to second time-scale charge carrier kinetics in hematite-titania nanocomposite photoanodes. J. Phys. Chem. Lett. 2015, 6, 2859–2864. [Google Scholar] [CrossRef] [PubMed]
- Skorb, K.V.; Antonouskaya, L.I.; Belyasova, N.A.; Shchukin, D.G.; Mohwald, H.; Sviridov, D.V. Antibacterial activity of thin film photocatalysts based on metal modified TiO2 and TiO2: In2O3 nanocomposite. Appl. Catal. B 2008, 84, 94–99. [Google Scholar] [CrossRef]
- Ahmad, W.; Noor, T.; Zeeshan, M. Effect of synthesis route on catalytic properties and performance of Co3O4/TiO2 for carbon monoxide and hydrocarbon oxidation under real engine operating conditions. Catal. Commun. 2017, 89, 19–24. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Zhao, Z.H.; Guo, W.B.; Qiu, J.C.; Mou, X.N.; Li, A.X.; Claverie, J.P.; Liu, H. NiO–TiO2 p-n heterostructured nanocables bridged by zero-bandgap rGO for highly efficient photocatalytic water splitting. Nano Energy 2015, 16, 207–217. [Google Scholar] [CrossRef]
- Zhou, W.J.; Yin, Z.Y.; Du, Y.P.; Huang, X.; Zeng, Z.Y.; Fan, Z.X.; Liu, H.; Wang, J.Y.; Zhang, H. Synthesis of few layer MoS2 nanosheet coated TiO2 nanobelt heterostructures for enhanced photocatalytic activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Zhang, X.; Zhang, N.; Tang, Z.R.; Xu, Y.J. Two-dimensional MoS2 nanosheet-coated Bi2S3 discoids: Synthesis, formation mechanism, and photocatalytic application. Langmuir 2015, 31, 4314–4322. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Yu, K.; Lei, X.; Guo, B.J.; Li, C.; Fu, H.; Zhu, Z.Q. Synthesis of the MoS2@CuO heterogeneous structure with improved photocatalysis performance and H2O adsorption analysis. Dalton Trans. 2015, 44, 10438–10447. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.Y.; Du, Y.L.; Feng, Q.L.; Ye, X.L.; Xie, H.; Xue, M.Y.; Wang, C.M. Synthesis of platinum nanoparticles by using molybdenum disulfide as a template and its application to enzyme-like catalysis. ChemCatChem 2014, 6, 1873–1876. [Google Scholar] [CrossRef]
- Zong, X.; Yan, H.J.; Wu, G.P.; Ma, G.J.; Wen, F.Y.; Wang, L.; Li, C. Enhancement of photocatalytic H2 evolution on CdS by loading MoS2 as cocatalyst under visible light irradiation. J. Am. Chem. Soc. 2008, 130, 7176–7177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.L.; Lin, B.Z.; Zhou, Y.; Sun, P.; Yao, Q.R.; Chen, Y.L.; Gao, B.F. Enhanced photocatalytic H2 evolution on ZnS loaded with graphene and MoS2 nanosheets as cocatalysts. J. Mater. Chem. A 2014, 2, 3819–3827. [Google Scholar] [CrossRef]
- Shen, M.; Yan, Z.P.; Yang, L.; Du, P.W.; Zhang, J.Y.; Xiang, B. MoS2 nanosheet/TiO2 nanowire hybrid nanostructures for enhanced visible-light photocatalytic activities. Chem. Commun. 2014, 50, 15447–15449. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.Y.; Zhang, H.C.; Zhu, W.; Yu, X.Y.; Kuang, Y.; Chang, Z.; Lei, X.D.; Sun, X.M. In situ fabrication of porous MoS2 thin films as high performance catalysts for electrochemical hydrogen evolution. Chem. Commun. 2013, 49, 7516–7518. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C.; Gao, P.; Yang, Y.R.; Yang, P.P.; Chen, Y.J.; Wang, Y.B. N-doped TiO2 nanobelts with coexposed (001) and (101) facets and their highly efficient visible-light-driven photocatalytic hydrogen production. ACS Appl. Mater. Interfaces 2016, 8, 18126–18131. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.Q.; Wang, J.; Tafen, D.N.; Wang, H.; Zheng, J.G.; Lewis, J.P.; Liu, X.G.; Leonard, S.S.; Manivannan, A. Shape enhanced photocatalytic activity of single-crystalline anatase TiO2 (101) nanobelts. J. Am. Chem. Soc. 2010, 132, 6679–6685. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Li, W.; Li, M.C.; Cui, P.; Chen, D.H.; Genenbach, T.; Chu, L.H.; Liu, H.Y.; Song, G.S. Glucose-assisted synthesis of the hierarchical TiO2 nanowire@MoS2 nanosheet nanocomposite and its synergistic lithium storage performance. J. Mater. Chem. A 2015, 3, 2762–2769. [Google Scholar] [CrossRef]
- Ke, J.; Liu, J.; Sun, H.Q.; Zhang, H.Y.; Duan, X.G.; Liang, P.; Li, X.Y.; Tade, M.; Liu, S.M.; Wang, S.B. Facile assembly of Bi2O3/Bi2S3/MoS2 n-p heterojunction with layered n-Bi2O3 and p-MoS2 for enhanced photocatalytic water oxidation and pollutant degradation. Appl. Catal. B 2017, 200, 47–55. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, B.; Wen, Z.H.; Cui, S.M.; Guo, X.R.; He, Z.; Chen, J.H. A 3D hybrid of layered MoS2/nitrogen-doped graphene nanosheet aerogels: An effective catalyst for hydrogen evolution in microbial electrolysis cells. J. Mater. Chem. A 2014, 2, 13795–13800. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, H.; Yang, X.R. Amorphous carbon supported MoS2 nanosheets as effective catalysts for electrocatalytic hydrogen evolution. Nanoscale 2014, 6, 10680–10685. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, M.; Zhang, H.M.; Liu, P.R.; Wang, Y.; Cao, J.; Zhao, H.J. Directly hydrothermal growth of ultrathin MoS2 nanostructured films as high performance counter electrodes for dye-sensitised solar cells. RSC Adv. 2014, 4, 21277–21283. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.S.; Jin, S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; He, J.F.; Liu, Q.H.; Yao, T.; Chen, L.; Yan, W.S.; Hu, F.C.; Jiang, Y.; Zhao, Y.D.; Hu, T.D.; et al. Vacancy-induced ferromagnetism of MoS2 nanosheets. J. Am. Chem. Soc. 2015, 137, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Ndokoye, P.; Ke, J.; Liu, J.; Zhao, Q.D.; Li, X.Y. l-Cysteine-modified gold nanostars for SERS-based copper ions detection in aqueous media. Langmuir 2014, 30, 13491–13497. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Li, X.Y.; Zhao, Q.D.; Hou, Y.; Chen, J.H. Ultrasensitive quantum dot fluorescence quenching assay for selective detection of mercury ions in drinking water. Sci. Rep. 2014, 4, 5624. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Ding, J.J.; Yao, S.W.; Wu, X.X.; Feng, Q.Q.; Wang, Z.H.; Geng, B.Y. High supercapacitor and adsorption behaviors of flower-like MoS2 nanostructures. J. Mater. Chem. A 2014, 2, 15958–15963. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Liu, Y.Y.; Jiang, T.; Huang, B.B.; Wang, Y.Y.; Zhang, X.Y.; Qin, X.Y.; Dai, Y. Enhancing visible light photocatalytic activity of TiO2 using a colorless molecule (2-methoxyethanol) due to hydrogen bond effect. Appl. Catal. B 2017, 200, 230–236. [Google Scholar] [CrossRef]
- Liu, J.M.; Han, L.; An, N.; Xing, L.; Cheng, L.; Yang, J.C.; Zhang, Q.C. Enhanced visible light photocatalytic activity of carbonate doped anatase TiO2 based on the electron withdrawing bidentate carboxylate linkage. Appl. Catal. B 2017, 202, 642–652. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.Z.; Song, Q.; Zhang, L.Q.; Song, X.J.; Wang, K.; Zhang, Y.F.; Li, J.; Wen, J.; Tian, J. Ag2O nanoparticle/TiO2 nanobelt heterostructures with remarkable photo-response and photocatalytic properties under UV, visible and near-infrared irradiation. Appl. Catal. B 2016, 198, 83–90. [Google Scholar] [CrossRef]
- Wang, C.C.; Du, X.D.; Guo, X.X.; Wang, P.; Zhang, J. Photocatalytic Cr(VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. B 2016, 193, 198–216. [Google Scholar] [CrossRef]
- Sun, J.J.; Li, X.Y.; Zhao, Q.D.; Ke, J.; Zhang, D.K. Novel V2O5/BiVO4/TiO2 nanocomposites with high visible light induced photocatalytic activity for the degradation of toluene. J. Phys. Chem. C 2014, 118, 10113–10121. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, M.; Chen, Q.G.; Fan, C.M.; Zhou, H.Y.; Xu, A.W. Novel one dimensional Bi2O3-Bi2WO6 p-n hierarchical heterojunction with enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 8517–8524. [Google Scholar] [CrossRef]










© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Li, Y.; Ke, J.; Wang, Z.; Xiao, H. Synergically Improving Light Harvesting and Charge Transportation of TiO2 Nanobelts by Deposition of MoS2 for Enhanced Photocatalytic Removal of Cr(VI). Catalysts 2017, 7, 30. https://0-doi-org.brum.beds.ac.uk/10.3390/catal7010030
Liu J, Li Y, Ke J, Wang Z, Xiao H. Synergically Improving Light Harvesting and Charge Transportation of TiO2 Nanobelts by Deposition of MoS2 for Enhanced Photocatalytic Removal of Cr(VI). Catalysts. 2017; 7(1):30. https://0-doi-org.brum.beds.ac.uk/10.3390/catal7010030
Chicago/Turabian StyleLiu, Jie, Ying Li, Jun Ke, Zhong Wang, and Huining Xiao. 2017. "Synergically Improving Light Harvesting and Charge Transportation of TiO2 Nanobelts by Deposition of MoS2 for Enhanced Photocatalytic Removal of Cr(VI)" Catalysts 7, no. 1: 30. https://0-doi-org.brum.beds.ac.uk/10.3390/catal7010030