Enhanced Catalytic Dechlorination of 1,2-Dichlorobenzene Using Ni/Pd Bimetallic Nanoparticles Prepared by a Pulsed Laser Ablation in Liquid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Analysis
2.2. Catalytic Dechlorination of 1,2-DCB Using Ni/Pd Bimetallic NPs
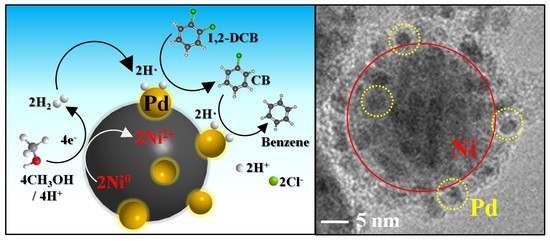
2.3. Mechanism of 1,2-DCB Degradation on the Ni/Pd Bimetallic NPs
3. Materials and Methods
3.1. Chemicals
3.2. PLAL Conditions
3.3. Preparation of Ni/Pd Bimetallic NPs
3.4. Dechlorination of 1,2-DCB
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gui, M.; Ormsbee, L.E.; Bhattacharyya, D. Reactive functionalized membranes for polychlorinated biphenyl degradation. Ind. Eng. Chem. Res. 2013, 52, 10430–10440. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Deng, S.; Xu, J.; Zhang, W.; Wu, M.; Wang, B.; Huang, J.; Yu, G. Highly active and stable Ni-Fe bimetal prepared by ball milling for catalytic hydrodechlorination of 4-chlorophenol. Environ. Sci. Technol. 2012, 46, 4576–4582. [Google Scholar] [CrossRef] [PubMed]
- Maire, J.; Joubert, A.; Kaifas, D.; Invernizzi, T.; Marduel, J.; Colombano, S.; Cazaux, D.; Marion, C.; Klein, P.Y.; Dumestre, A.; et al. Assessment of flushing methods for the removal of heavy chlorinated compounds DNAPL in an alluvial aquifer. Sci. Total Environ. 2018, 612, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zuo, S.; Zhou, R. Synergistic catalytic effect of (Ce,Cr)xO2 and HZSM-5 for elimination of chlorinated organic pollutants. Chem. Eng. J. 2017, 323, 160–170. [Google Scholar] [CrossRef]
- Jalil, A.A.; Triwahyono, S.; Razali, N.A.M.; Hairom, N.H.H.; Idris, A.; Muhid, M.N.M.; Ismail, A.; Yahaya, N.A.M.; Ahmad, N.A.L.; Dzinun, H. Complete electrochemical dechlorination of chlorobenzenes in the presence of various arene mediators. J. Hazard. Mater. 2010, 174, 581–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano, M.; Guarín, F.; Aristizábal, B.; Villa, A.L.; González, L.M. Catalytic activity and stability of Pd/Co catalysts in simultaneous selective catalytic reduction of NOx with methane and oxidation of o-dichlorobenzene. Catal. Today 2017, 296, 105–117. [Google Scholar] [CrossRef]
- Hosseinkhani, B.; Nuzzo, A.; Zanaroli, G.; Fava, F.; Boon, N. Assessment of catalytic dechlorination activity of suspended and immobilized bio-Pd NPs in different marine conditions. Appl. Catal. B 2015, 168–169, 62–67. [Google Scholar] [CrossRef]
- Kim, N.R.; Shin, K.; Jung, I.; Shim, M.; Lee, H.M. Ag-Cu Bimetallic Nanoparticles with Enhanced Resistance to Oxidation: A Combined Experimental and Theoretical Study. J. Phys. Chem. C 2014, 118, 26324–26331. [Google Scholar] [CrossRef]
- Dutta, S.; Ray, C.; Sarkar, S.; Roy, A.; Sahoo, R.; Pal, T. Facile Synthesis of Bimetallic Au-Pt, Pd-Pt, and Au-Pd Nanostructures: Enhanced Catalytic Performance of Pd-Pt Analogue towards Fuel Cell Application and Electrochemical Sensing. Electrochim. Acta 2015, 180, 1075–1084. [Google Scholar] [CrossRef]
- Chang, L.; Li, Y. One-step encapsulation of Pt-Co bimetallic nanoparticles within MOFs for advanced room temperature nanocatalysis. Mol. Catal. 2017, 433, 77–83. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, Z.; Deng, J.; Zhang, C.; Cheng, Y.; Hou, K.; Zhang, L.; Tang, L.; Zeng, G. Physicochemical transformation of Fe/Ni bimetallic nanoparticles during aging in simulated groundwater and the consequent effect on contaminant removal. Water Res. 2018, 129, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Gołąbiewska, A.; Lisowski, W.; Jarek, M.; Nowaczyk, G.; Michalska, M.; Jurga, S.; Zaleska-Medynska, A. The effect of metals content on the photocatalytic activity of TiO2 modified by Pt/Au bimetallic nanoparticles prepared by sol-gel method. Mol. Catal. 2017, 442, 154–163. [Google Scholar] [CrossRef]
- Kannan, P.; Yoon, C.S.; Yi, S.C.; Lee, S.Y.; Kim, D.H. Shape-controlled synthesis of gold-nickel bimetallic nanoparticles and their electrocatalytic properties. Mater. Chem. Phys. 2015, 156, 1–8. [Google Scholar] [CrossRef]
- Lin, J.; Chen, J.; Su, W. Rhodium-cobalt bimetallic nanoparticles: A catalyst for selective hydrogenation of unsaturated carbon-carbon bonds with hydrous hydrazine. Adv. Synth. Catal. 2013, 355, 41–46. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Cheng, D. Component-dependent electrocatalytic activity of PdCu bimetallic nanoparticles for hydrogen evolution reaction. Electrochim. Acta 2017, 246, 572–579. [Google Scholar]
- Chen, S.S.; Yang, Z.Z.; Wang, A.J.; Fang, K.M.; Feng, J.J. Facile synthesis of bimetallic gold-palladium nanocrystals as effective and durable advanced catalysts for improved electrocatalytic performances of ethylene glycol and glycerol oxidation. J. Colloid Interface Sci. 2018, 509, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Pino, N.; Sitthisa, S.; Tan, Q.; Souza, T.; López, D.; Resasco, D.E. Structure, activity, and selectivity of bimetallic Pd-Fe/SiO2 and Pd-Fe/Γ-Al2O3 catalysts for the conversion of furfural. J. Catal. 2017, 350, 30–40. [Google Scholar] [CrossRef]
- Tuo, Y.; Liu, G.; Dong, B.; Zhou, J.; Wang, A.; Wang, J.; Jin, R.; Lv, H.; Dou, Z.; Huang, W. Microbial synthesis of Pd/Fe3O4, Au/Fe3O4 and PdAu/Fe3O4 nanocomposites for catalytic reduction of nitroaromatic compounds. Sci. Rep. 2015, 5, 13515. [Google Scholar] [CrossRef] [PubMed]
- Bedia, J.; Arevalo-Bastante, A.; Grau, J.M.; Dosso, L.A.; Rodriguez, J.J.; Mayoral, A.; Diaz, I.; Gómez-Sainero, L.M. Effect of the Pt–Pd molar ratio in bimetallic catalysts supported on sulfated zirconia on the gas-phase hydrodechlorination of chloromethanes. J. Catal. 2017, 352, 562–571. [Google Scholar] [CrossRef]
- Fang, D.; Li, W.; Zhao, J.; Liu, S.; Ma, X.; Xu, J.; Xia, C. Catalytic hydrodechlorination of 4-chlorophenol over a series of Pd-Cu/γ-Al2O3 bimetallic catalysts. RSC Adv. 2014, 4, 59204–59210. [Google Scholar] [CrossRef]
- Karanjit, S.; Jinasan, A.; Samsook, E.; Dhital, R.N.; Motomiya, K.; Sato, Y.; Tohji, K.; Sakurai, H. Significant stabilization of palladium by gold in the bimetallic nanocatalyst leading to an enhanced activity in the hydrodechlorination of aryl chlorides. Chem. Commun. 2015, 51, 12724–12727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Liu, X.; Lowry, G.V.; Cao, Z.; Zhao, H.; Zhou, J.L.; Xu, X. Dechlorination Mechanism of 2,4-Dichlorophenol by Magnetic MWCNTs Supported Pd/Fe Nanohybrids: Rapid Adsorption, Gradual Dechlorination, and Desorption of Phenol. ACS Appl. Mater. Interfaces 2016, 8, 7333–7342. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.; Gu, X.; Lu, S.; Ahmad, A.; Naqvi, M.; Farooq, U.; Zhang, X.; Fu, X.; Miao, Z.; Xue, Y. Efficient transformation of trichloroethylene activated through sodium percarbonate using heterogeneous zeolite supported nano zero valent iron-copper bimetallic composite. Chem. Eng. J. 2017, 308, 396–407. [Google Scholar] [CrossRef]
- Danish, M.; Gu, X.; Lu, S.; Farooq, U.; Zaman, W.Q.; Fu, X.; Miao, Z.; Brusseau, M.L.; Ahmad, A.; Naqvi, M. An efficient catalytic degradation of trichloroethene in a percarbonate system catalyzed by ultra-fine heterogeneous zeolite supported zero valent iron-nickel bimetallic composite. Appl. Phys. A 2017, 531, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallikarjuna, K.; Kim, H. Synthesis and characterization of highly active Cu/Pd bimetallic nanostructures. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 194–200. [Google Scholar] [CrossRef]
- Zhao, D.; Li, M.; Zhang, D.; Baig, S.A.; Xu, X. Reductive dechlorination of 2,4-dichlorophenol by Pd/Fe nanoparticles prepared in the presence of ultrasonic irradiation. Ultrason. Sonochem. 2013, 20, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Feng, Q. Fabrication of bimetallic Ag/Fe immobilized on modified biochar for removal of carbon tetrachloride. J. Environ. Sci. 2017, 54, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Feng, Q.; Yang, H.; Alam, E.; Gao, B.; Gu, D. Modified biochar supported Ag/Fe nanoparticles used for removal of cephalexin in solution: Characterization, kinetics and mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2017, 517, 63–71. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Lisowski, W.; Zaleska-Medynska, A. Preparation, characterization and photocatalytic activity of TiO2 microspheres decorated by bimetallic nanoparticles. J. Mol. Catal. A Chem. 2016, 424, 241–253. [Google Scholar] [CrossRef]
- Peng, Z.; Yu, Z.; Wang, L.; Hou, Y.; Shi, Y.; Wu, L.; Li, Z. Facile synthesis of Pd-Fe nanoparticles modified Ni foam electrode and its behaviors in electrochemical reduction of tetrabromobisphenol A. Mater. Lett. 2016, 166, 300–303. [Google Scholar] [CrossRef]
- Qin, N.; Zhang, Y.; Zhou, H.; Geng, Z.; Liu, G.; Zhang, Y.; Zhao, H.; Wang, G. Enhanced removal of trace Cr(VI) from neutral and alkaline aqueous solution by FeCo bimetallic nanoparticles. J. Colloid Interface Sci. 2016, 472, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, C.; Chen, H.; Ma, J. Characterization and evaluation of catalytic dechlorination activity of Pd/Fe bimetallic nanoparticles. Ind. Eng. Chem. Res. 2008, 47, 8645–8651. [Google Scholar] [CrossRef]
- Amendola, V.; Scaramuzza, S.; Carraro, F.; Cattaruzza, E. Formation of alloy nanoparticles by laser ablation of Au/Fe multilayer films in liquid environment. J. Colloid Interface Sci. 2017, 489, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Choi, M.Y. Specific Solvent Produces Specific Phase Ni Nanoparticles: A Pulsed Laser Ablation in Solvents. J. Phys. Chem. C 2014, 118, 14647–14654. [Google Scholar] [CrossRef]
- Sivasakthi, P.; Ramesh Bapu, G.N.K.; Chandrasekaran, M.; Sreejakumari, S.S. Synthesis of a super-hydrophobic Ni-ITO nanocomposite with pine-cone and spherical shaped micro-nanoarchitectures by pulse electrodeposition and its electrocatalytic application. RSC Adv. 2016, 6, 44766–44773. [Google Scholar] [CrossRef]
- Sengupta, D.; Saha, J.; De, G.; Basu, B. Pd/Cu bimetallic nanoparticles embedded in macroporous ion-exchange resins: An excellent heterogeneous catalyst for the Sonogashira reaction. J. Mater. Chem. A 2014, 2, 3986–3992. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Dasgupta, S. Trimetallic NiFePd nanoalloy catalysed hydrogen generation from alkaline hydrous hydrazine and sodium borohydride at room temperature. J. Mater. Chem. A 2015, 3, 24371–24378. [Google Scholar] [CrossRef]
- Maity, M.; Maitra, U. An easily prepared palladium-hydrogel nanocomposite catalyst for C-C coupling reactions. J. Mater. Chem. A 2014, 2, 18952–18958. [Google Scholar] [CrossRef]
- Zhou, T.; Li, Y.; Lim, T.T. Catalytic hydrodechlorination of chlorophenols by Pd/Fe nanoparticles: Comparisons with other bimetallic systems, kinetics and mechanism. Sep. Purif. Technol. 2010, 76, 206–214. [Google Scholar] [CrossRef]
- Nagpal, V.; Bokare, A.D.; Chikate, R.C.; Rode, C.V.; Paknikar, K.M. Reductive dechlorination of γ-hexachlorocyclohexane using Fe-Pd bimetallic nanoparticles. J. Hazard. Mater. 2010, 175, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Jung, H.J.; Je, M.; Choi, H.C.; Choi, M.Y. Enhanced dechlorination of m-DCB using iron-graphite/palladium (Fe-C/Pd) nanoparticles produced by pulsed laser ablation in liquid. Chemosphere 2016, 155, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, H.; Chen, Q.; Huang, Z. Bimetallic Au-decorated Pd catalyst for the liquid phase hydrodechlorination of 2,4-dichlorophenol. Appl. Surf. Sci. 2016, 387, 588–594. [Google Scholar] [CrossRef]
- Seshu Babu, N.; Lingaiah, N.; Sai Prasad, P.S. Characterization and reactivity of Al2O3 supported Pd-Ni bimetallic catalysts for hydrodechlorination of chlorobenzene. Appl. Catal. B 2012, 111–112, 309–316. [Google Scholar] [CrossRef]
- Kim, P.; Kim, Y.; Kim, H.; Song, I.K.; Yi, J. Preparation, characterization, and catalytic activity of NiMg catalysts supported on mesoporous alumina for hydrodechlorination of o-dichlorobenzene. J. Mol. Catal. A Chem. 2005, 231, 247–254. [Google Scholar] [CrossRef]
- Schrick, B.; Blough, J.L.; Jones, A.D.; Mallouk, T.E. Hydrodechlorination of trichloroethylene to hydrocarbons using bimetallic nickel-iron nanoparticles. Chem. Mater. 2002, 14, 5140–5147. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Horita, J.; Yan, W. Trichloroethene hydrodechlorination by Pd-Fe bimetallic nanoparticles: Solute-induced catalyst deactivation analyzed by carbon isotope fractionation. Appl. Catal. B 2016, 188, 77–86. [Google Scholar] [CrossRef]
- Shen, W.; Mu, Y.; Wang, B.; Ai, Z.; Zhang, L. Enhanced aerobic degradation of 4-chlorophenol with iron-nickel nanoparticles. Appl. Surf. Sci. 2017, 393, 316–324. [Google Scholar] [CrossRef]
- Xu, J.; Lv, X.; Li, J.; Li, Y.; Shen, L.; Zhou, H.; Xu, X. Simultaneous adsorption and dechlorination of 2,4-dichlorophenol by Pd/Fe nanoparticles with multi-walled carbon nanotube support. J. Hazard. Mater. 2012, 225–226, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Lo, S.L.; Liou, Y.H. Dechlorination of trichloroethylene in aqueous solution by noble metal-modified iron. J. Hazard. Mater. B 2004, 116, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lim, T.T. Pathways and kinetics of carbon tetrachloride and chloroform reductions by nano-scale Fe and Fe/Ni particles: Comparison with commercial micro-scale Fe and Zn. Chemosphere 2005, 59, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Wang, Z.L.; Pal, T. Redox Catalytic Properties of Palladium Nanoparticles: Surfactant and Electron Donor-Acceptor Effects. Langmuir 2000, 16, 2457–2463. [Google Scholar] [CrossRef]
- Castegnaro, M.V.; Kilian, A.S.; Baibich, I.M.; Alves, M.C.M.; Morais, J. On the Reactivity of Carbon Supported Pd Nanoparticles during NO Reduction: Unraveling a Metal–Support Redox Interaction. Langmuir 2013, 29, 7125–7133. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ahn, A.; Choi, M.Y. Direct observation of aluminium ions produced via pulsed laser ablation in liquid: A ‘turn-on’ fluorescence study. Phys. Chem. Chem. Phys. 2012, 14, 15677–15681. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.J.; Lee, S.J.; Koutavarapu, R.; Kim, S.K.; Choi, H.C.; Choi, M.Y. Enhanced Catalytic Dechlorination of 1,2-Dichlorobenzene Using Ni/Pd Bimetallic Nanoparticles Prepared by a Pulsed Laser Ablation in Liquid. Catalysts 2018, 8, 390. https://0-doi-org.brum.beds.ac.uk/10.3390/catal8090390
Jung HJ, Lee SJ, Koutavarapu R, Kim SK, Choi HC, Choi MY. Enhanced Catalytic Dechlorination of 1,2-Dichlorobenzene Using Ni/Pd Bimetallic Nanoparticles Prepared by a Pulsed Laser Ablation in Liquid. Catalysts. 2018; 8(9):390. https://0-doi-org.brum.beds.ac.uk/10.3390/catal8090390
Chicago/Turabian StyleJung, Hyeon Jin, Seung Jun Lee, Ravindranadh Koutavarapu, Sung Kuk Kim, Hyun Chul Choi, and Myong Yong Choi. 2018. "Enhanced Catalytic Dechlorination of 1,2-Dichlorobenzene Using Ni/Pd Bimetallic Nanoparticles Prepared by a Pulsed Laser Ablation in Liquid" Catalysts 8, no. 9: 390. https://0-doi-org.brum.beds.ac.uk/10.3390/catal8090390