ZIF-8 Metal Organic Framework for the Conversion of Glucose to Fructose and 5-Hydroxymethyl Furfural
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation of Catalysts
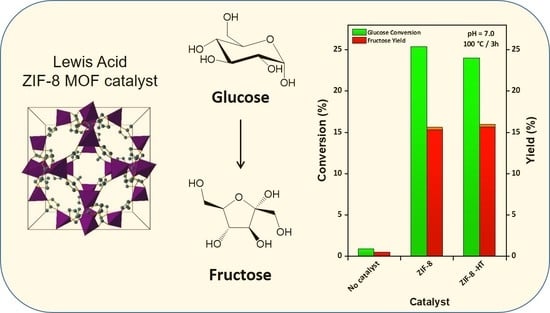
2.2. Catalytic Activity
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wheeldon, I.; Christopher, P.; Blanch, H. Integration of heterogeneous and biochemical catalysis for production of fuels and chemicals from biomass. Curr. Opin. Biotechnol. 2017, 45, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Romo, J.E.; Bollar, N.V.; Zimmermann, C.J.; Wettstein, S.G. Conversion of Sugars and Biomass to Furans Using Heterogeneous Catalysts in Biphasic Solvent Systems. ChemCatChem 2018, 10, 4805–4816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavuz, E.; Cherkasov, N.; Degirmenci, V. Acid and base catalysed reactions in one pot with site-isolated polyHIPE catalysts. RSC Adv. 2019, 9, 8175–8183. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Pidko, E.A.; Degirmenci, V.; van Santen, R.A.; Hensen, E.J.M. Glucose Activation by Transient Cr2+ Dimers. Angew. Chem. Int. Ed. 2010, 49, 2530–2534. [Google Scholar] [CrossRef] [PubMed]
- Pidko, E.A.; Degirmenci, V.; Hensen, E.J.M. On the Mechanism of Lewis Acid Catalyzed Glucose Transformations in Ionic Liquids. ChemCatChem 2012, 4, 1263–1271. [Google Scholar] [CrossRef]
- Pidko, E.A.; Degirmenci, V.; van Santen, R.A.; Hensen, E.J.M. Coordination Properties of Ionic Liquid-Mediated Chromium(II) and Copper(II) Chlorides and Their Complexes with Glucose. Inorg. Chem. 2010, 49, 10081–10091. [Google Scholar] [CrossRef]
- Degirmenci, V.; Hensen, E.J.M. Development of a Heterogeneous Catalyst for Lignocellulosic Biomass Conversion: Glucose Dehydration by Metal Chlorides in a Silica-Supported Ionic Liquid Layer. Environ. Prog. Sustain. Energy 2014, 33, 657–662. [Google Scholar] [CrossRef]
- Degirmenci, V.; Pidko, E.A.; Magusin, P.C.M.M.; Hensen, E.J.M. Towards a Selective Heterogeneous Catalyst for Glucose Dehydration to 5-Hydroxymethylfurfural in Water: CrCl2 Catalysis in a Thin Immobilized Ionic Liquid Layer. ChemCatChem 2011, 3, 969–972. [Google Scholar] [CrossRef]
- Davis, M.E. Heterogeneous Catalysis for the Conversion of Sugars into Polymers. Top. Catal. 2015, 58, 405–409. [Google Scholar] [CrossRef]
- Moliner, M.; Román-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 2010, 107, 6164–6168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabbeigi, N.; Torres, A.I.; Lew, C.M.; Elyassi, B.; Ren, L.; Wang, Z.; Je Cho, H.; Fan, W.; Daoutidis, P.; Tsapatsis, M. On the kinetics of the isomerization of glucose to fructose using Sn-Beta. Chem. Eng. Sci. 2014, 116, 235–242. [Google Scholar] [CrossRef]
- Toftgaard Pedersen, A.; Ringborg, R.; Grotkjær, T.; Pedersen, S.; Woodley, J.M. Synthesis of 5-hydroxymethylfurfural (HMF) by acid catalyzed dehydration of glucose–fructose mixtures. Chem. Eng. J. 2015, 273, 455–464. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Davis, M.E. Activation of Carbonyl-Containing Molecules with Solid Lewis Acids in Aqueous Media. ACS Catal. 2011, 1, 1566–1580. [Google Scholar] [CrossRef]
- Gounder, R.; Davis, M.E. Monosaccharide and disaccharide isomerization over Lewis acid sites in hydrophobic and hydrophilic molecular sieves. J. Catal. 2013, 308, 176–188. [Google Scholar] [CrossRef]
- Caratzoulas, S.; Davis, M.E.; Gorte, R.J.; Gounder, R.; Lobo, R.F.; Nikolakis, V.; Sandler, S.I.; Snyder, M.A.; Tsapatsis, M.; Vlachos, D.G. Challenges of and Insights into Acid-Catalyzed Transformations of Sugars. J. Phys. Chem. C 2014, 118, 22815–22833. [Google Scholar] [CrossRef] [Green Version]
- Román-Leshkov, Y.; Moliner, M.; Labinger, J.A.; Davis, M.E. Mechanism of Glucose Isomerization Using a Solid Lewis Acid Catalyst in Water. Angew. Chem. Int. Ed. 2010, 49, 8954–8957. [Google Scholar] [CrossRef] [Green Version]
- Rai, N.; Caratzoulas, S.; Vlachos, D.G. Role of Silanol Group in Sn-Beta Zeolite for Glucose Isomerization and Epimerization Reactions. ACS Catal. 2013, 3, 2294–2298. [Google Scholar] [CrossRef]
- Osmundsen, C.M.; Holm, M.S.; Dahl, S.; Taarning, E. Tin-containing silicates: Structure-activity relations. Proc. R. Soc. A 2012, 468, 2000–2016. [Google Scholar] [CrossRef]
- Zhang, Y.; Degirmenci, V.; Li, C.; Hensen, E.J.M. Phosphotungstic Acid Encapsulated in Metal-Organic Framework as Catalysts for Carbohydrate Dehydration to 5-Hydroxymethylfurfural. ChemSusChem 2011, 4, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, R.; Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Cherkasov, N.; Walker, M.; Kashtiban, R.J.; Krisnandi, Y.K.; Degirmenci, V.; Walton, R.I. Replacement of Chromium by Non-Toxic Metals in Lewis-Acid MOFs: Assessment of Stability as Glucose Conversion Catalysts. Catalysts 2019, 9, 437. [Google Scholar] [CrossRef]
- Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Walton, R.I.; Degirmenci, V. Exceptionally Efficient and Recyclable Heterogeneous Metal–Organic Framework Catalyst for Glucose Isomerization in Water. ChemCatChem 2018, 10, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z. Development of ZIF-8 membranes: Opportunities and challenges for commercial applications. Curr. Opin. Chem. Eng. 2018, 20, 78–85. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Kolmykov, O.; Chebbat, N.; Commenge, J.M.; Medjahdi, G.; Schneider, R. ZIF-8 nanoparticles as an efficient and reusable catalyst for the Knoevenagel synthesis of cyanoacrylates and 3-cyanocoumarins. Tetrahedron Lett. 2016, 57, 5885–5888. [Google Scholar] [CrossRef]
- Sun, C.Y.; Qin, C.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Su, Z.M.; Huang, P.; Wang, C.G.; Wang, E.B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef]
- Gong, X.; Wang, Y.; Kuang, T. ZIF-8-Based Membranes for Carbon Dioxide Capture and Separation. ACS Sustain. Chem. Eng. 2017, 5, 11204–11214. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Liu, J.; Yan, H. The applications of zeolitic imidazolate framework-8 in electrical energy storage devices: A review. J. Mater. Sci. Mater. Electron. 2017, 28, 7532–7543. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.N.; Li, Z.; Zhang, B.; Zhu, M.; Hu, X.; Zhang, Y.; Li, F. Zeolitic Imidazolate Framework-8 with High Efficiency in Trace Arsenate Adsorption and Removal from Water. J. Phys. Chem. C 2014, 118, 27382–27387. [Google Scholar] [CrossRef]
- Bleith, T.; Deng, Q.H.; Wadepohl, H.; Gade, L.H. Radical Changes in Lewis Acid Catalysis: Matching Metal and Substrate. Angew. Chem. Int. Ed. 2016, 55, 7852–7856. [Google Scholar] [CrossRef] [PubMed]
- Orazov, M.; Davis, M.E. Catalysis by framework zinc in silica-based molecular sieves. Chem. Sci. 2016, 7, 2264–2274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, B.; Sant, M.; Demontis, P.; Suffritti, G.B. Force Field for Molecular Dynamics Computations in Flexible ZIF-8 Framework. J. Phys. Chem. C 2012, 116, 933–938. [Google Scholar] [CrossRef]
- Moggach, S.A.; Bennett, T.D.; Cheetham, A.K. The Effect of Pressure on ZIF-8: Increasing Pore Size with Pressure and the Formation of a High-Pressure Phase at 1.47 GPa. Angew. Chem. Int. Ed. 2009, 48, 7087–7089. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, H.; Udovic, T.J.; Rush, J.J.; Yildirim, T. Quasi-Free Methyl Rotation in Zeolitic Imidazolate Framework-8. J. Phys. Chem. A 2008, 112, 12602–12606. [Google Scholar] [CrossRef] [PubMed]
- Liédana, N.; Galve, A.; Rubio, C.; Téllez, C.; Coronas, J. CAF@ZIF-8: One-Step Encapsulation of Caffeine in MOF. ACS Appl. Mater. Interfaces 2012, 4, 5016–5021. [Google Scholar] [CrossRef] [PubMed]
- Haldoupis, E.; Watanabe, T.; Nair, S.; Sholl, D.S. Quantifying Large Effects of Framework Flexibility on Diffusion in MOFs: CH4 and CO2 in ZIF-8. ChemPhysChem 2012, 13, 3449–3452. [Google Scholar] [CrossRef]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef]
- Ordoñez, M.J.C.; Balkus, K.J.; Ferraris, J.P.; Musselman, I.H. Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes. J. Membr. Sci. 2010, 361, 28–37. [Google Scholar] [CrossRef]
- Butova, V.V.; Budnyk, A.P.; Bulanova, E.A.; Lamberti, C.; Soldatov, A.V. Hydrothermal synthesis of high surface area ZIF-8 with minimal use of TEA. Solid State Sci. 2017, 69, 13–21. [Google Scholar] [CrossRef]
- Munn, A.S.; Dunne, P.W.; Tang, S.V.Y.; Lester, E.H. Large-scale continuous hydrothermal production and activation of ZIF-8. Chem. Commun. 2015, 51, 12811–12814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Srinivas, D.; Bhogeswararao, S.; Ratnasamy, P.; Carreon, M.A. Catalytic activity of ZIF-8 in the synthesis of styrene carbonate from CO2 and styrene oxide. Catal. Commun. 2013, 32, 36–40. [Google Scholar] [CrossRef]
- Chizallet, C.; Bats, N. External Surface of Zeolite Imidazolate Frameworks Viewed Ab Initio: Multifunctionality at the Organic−Inorganic Interface. J. Phys. Chem. Lett. 2010, 1, 349–353. [Google Scholar] [CrossRef]
- Zhang, K.; Lively, R.P.; Zhang, C.; Koros, W.J.; Chance, R.R. Investigating the Intrinsic Ethanol/Water Separation Capability of ZIF-8: An Adsorption and Diffusion Study. J. Phys. Chem. C 2013, 117, 7214–7225. [Google Scholar] [CrossRef]
- van der Graaff, W.N.P.; Tempelman, C.H.L.; Li, G.; Mezari, B.; Kosinov, N.; Pidko, E.A.; Hensen, E.J.M. Competitive Adsorption of Substrate and Solvent in Sn-Beta Zeolite During Sugar Isomerization. ChemSusChem 2016, 9, 3145–3149. [Google Scholar] [CrossRef] [PubMed]
- van der Graaff, W.N.P.; Tempelman, C.H.L.; Pidko, E.A.; Hensen, E.J.M. Influence of pore topology on synthesis and reactivity of Sn-modified zeolite catalysts for carbohydrate conversions. Catal. Sci. Technol. 2017, 7, 3151–3162. [Google Scholar] [CrossRef] [Green Version]
- Gounder, R.; Davis, M.E. Beyond shape selective catalysis with zeolites: Hydrophobic void spaces in zeolites enable catalysis in liquid water. AlChE J. 2013, 59, 3349–3358. [Google Scholar] [CrossRef]
- van der Graaff, W.N.P.; Tempelman, C.H.L.; Hendriks, F.C.; Ruiz-Martinez, J.; Bals, S.; Weckhuysen, B.M.; Pidko, E.A.; Hensen, E.J.M. Deactivation of Sn-Beta during carbohydrate conversion. Appl. Catal. A Gen. 2018, 564, 113–122. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Toby, B. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]









| Catalyst | FWHM 1 (Degrees) | a (Å) | Volume (Å3) | d (nm) |
|---|---|---|---|---|
| ZIF-8 | 0.20 | 17.0288(5) | 4937.9(5) | 34.9 ± 4.8 |
| ZIF-8-HT | 0.14 | 17.0500(3) | 4956.5(3) | 45.2 ± 11.4 |
| Catalyst | SBET 1 (m2·g−1) | VTotal 2 (cm3·g−1) | VMeso 3 (cm3·g−1) | VMicro 4 (cm3·g−1) | Pore Size 5 (Å) |
|---|---|---|---|---|---|
| ZIF-8 | 1351 | 0.70 | 0.05 | 0.54 | 7.3 |
| ZIF-8-HT | 1967 | 0.83 | - | 0.83 | 6.6 |
| Catalyst | pH 1 | Temperature (°C) | Glucose Conversion (%) | Fructose Yield (%) | Mannose Yield (%) | HMF Yield (%) |
|---|---|---|---|---|---|---|
| No catalyst | 7.0 | 100 | 0.8 | 0.4 | 0 | 0 |
| 7.0 | 140 | 5.6 | 0.8 | 0 | 2.1 | |
| 1.0 | 100 | 5.7 | 0 | 0 | 0.2 | |
| 1.0 | 140 | 18.4 | 0 | 0 | 4.2 | |
| ZnCl2 | 7.0 | 100 | 5.6 | 2.6 | 0 | 0 |
| 7.0 | 140 | 28.3 | 7.7 | 0.1 | 8.7 | |
| 1.0 | 100 | 5.5 | 0 | 0 | 0.2 | |
| 1.0 | 140 | 21.0 | 0 | 0 | 4.4 | |
| ZIF-8 | 7.0 | 100 | 25.3 | 15.3 | 0.3 | 0 |
| 7.0 | 140 | 84.2 | 12.1 | 2.6 | 1.2 | |
| 1.0 | 100 | 11.9 | 5.4 | 0 | 0 | |
| 1.0 | 140 | 72.6 | 17.0 | 2.8 | 2.6 | |
| ZIF-8-HT | 7.0 | 100 | 24.0 | 15.6 | 0.3 | 0 |
| 7.0 | 140 | 82.3 | 12.1 | 2.6 | 1.2 | |
| 1.0 | 100 | 9.4 | 5.1 | 0 | 0 | |
| 1.0 | 140 | 74.4 | 16.7 | 3.0 | 2.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oozeerally, R.; Ramkhelawan, S.D.K.; Burnett, D.L.; Tempelman, C.H.L.; Degirmenci, V. ZIF-8 Metal Organic Framework for the Conversion of Glucose to Fructose and 5-Hydroxymethyl Furfural. Catalysts 2019, 9, 812. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9100812
Oozeerally R, Ramkhelawan SDK, Burnett DL, Tempelman CHL, Degirmenci V. ZIF-8 Metal Organic Framework for the Conversion of Glucose to Fructose and 5-Hydroxymethyl Furfural. Catalysts. 2019; 9(10):812. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9100812
Chicago/Turabian StyleOozeerally, Ryan, Shivendra D. K. Ramkhelawan, David L. Burnett, Christiaan H. L. Tempelman, and Volkan Degirmenci. 2019. "ZIF-8 Metal Organic Framework for the Conversion of Glucose to Fructose and 5-Hydroxymethyl Furfural" Catalysts 9, no. 10: 812. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9100812