Catalytic Oxidation of NO over LaCo1−xBxO3 (B = Mn, Ni) Perovskites for Nitric Acid Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterisation
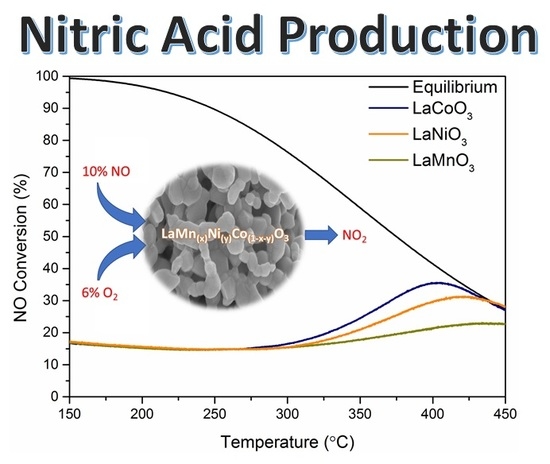
2.2. NO Oxidation Activity
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterisation
3.3. Catalyst Activity Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baulch, D.; Drysdale, D.; Horne, D. Homogeneous Gas Phase Reactions of the H2-N2-O2 System; CRC Press: Boca Raton, FL, USA, 1973; pp. 285–300. [Google Scholar]
- Honti, G. The Nitrogen Industry; Akademiai Kiado: Budapest, Hungary, 1976; pp. 400–413. [Google Scholar]
- Klingelhoefer, W.C. Nitric Oxide Oxidation. US2115173 A, 26 April 1938. [Google Scholar]
- Grande, C.A.; Andreassen, K.A.; Cavka, J.H.; Waller, D.; Lorentsen, O.-A.; Øien, H.; Zander, H.-J.; Poulston, S.; García, S.; Modeshia, D. Process intensification in nitric acid plants by catalytic oxidation of nitric oxide. Ind. Eng. Chem. Res. 2018, 57, 10180–10186. [Google Scholar] [CrossRef]
- Salman, A.u.R.; Enger, B.C.; Auvray, X.; Lødeng, R.; Menon, M.; Waller, D.; Rønning, M. Catalytic oxidation of NO to NO2 for nitric acid production over a Pt/Al2O3 catalyst. Appl. Catal. A Gen. 2018, 564, 142–146. [Google Scholar] [CrossRef]
- Russell, A.; Epling, W.S. Diesel oxidation catalysts. Catal. Rev. 2011, 53, 337–423. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, Z.; Li, X. Catalytic oxidation of nitric oxide (NO) over different catalysts: An overview. Catal. Sci. Technol. 2017, 7, 3440–3452. [Google Scholar] [CrossRef]
- Auvray, X.; Olsson, L. Stability and activity of Pd-, Pt- and Pd–Pt catalysts supported on alumina for NO oxidation. Appl. Catal. B Environ. 2015, 168–169, 342–352. [Google Scholar] [CrossRef]
- Auvray, X.; Pingel, T.; Olsson, E.; Olsson, L. The effect gas composition during thermal aging on the dispersion and NO oxidation activity over Pt/Al2O3 catalysts. Appl. Catal. B Environ. 2013, 129, 517–527. [Google Scholar] [CrossRef]
- Després, J.; Elsener, M.; Koebel, M.; Kröcher, O.; Schnyder, B.; Wokaun, A. Catalytic oxidation of nitrogen monoxide over Pt/SiO2. Appl. Catal. B Environ. 2004, 50, 73–82. [Google Scholar] [CrossRef]
- Zhu, J.; Thomas, A. Perovskite-type mixed oxides as catalytic material for NO removal. Appl. Catal. B Environ. 2009, 92, 225–233. [Google Scholar] [CrossRef]
- Onrubia, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Key factors in Sr-doped LaBO3 (B=Co or Mn) perovskites for NO oxidation in efficient diesel exhaust purification. Appl. Catal. B Environ. 2017, 213, 198–210. [Google Scholar] [CrossRef]
- Zhong, S.; Sun, Y.; Xin, H.; Yang, C.; Chen, L.; Li, X. NO oxidation over Ni–Co perovskite catalysts. Chem. Eng. J. 2015, 275, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-doped perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, C.; He, H.; Yu, Y.; Teraoka, Y. Catalytic oxidation of nitrogen monoxide over La1−xCexCoO3 perovskites. Catal. Today 2007, 126, 400–405. [Google Scholar] [CrossRef]
- Wang, J.; Su, Y.; Wang, X.; Chen, J.; Zhao, Z.; Shen, M. The effect of partial substitution of Co in LaMnO3 synthesized by sol–gel methods for NO oxidation. Catal. Commun. 2012, 25, 106–109. [Google Scholar] [CrossRef]
- Taguchi, H.; Matsu-ura, S.-i.; Nagao, M.; Choso, T.; Tabata, K. Synthesis of LaMnO3+δ by firing gels using citric acid. J. Solid State Chem. 1997, 129, 60–65. [Google Scholar] [CrossRef]
- Taguchi, H.; Yamada, S.; Nagao, M.; Ichikawa, Y.; Tabata, K. Surface characterization of LaCoO3 synthesized using citric acid. Mater. Res. Bull. 2002, 37, 69–76. [Google Scholar] [CrossRef]
- Alifanti, M.; Auer, R.; Kirchnerova, J.; Thyrion, F.; Grange, P.; Delmon, B. Activity in methane combustion and sensitivity to sulfur poisoning of La1−xCexMn1−yCoyO3 perovskite oxides. Appl. Catal. B Environ. 2003, 41, 71–81. [Google Scholar] [CrossRef]
- Silva, C.R.B.; da Conceição, L.; Ribeiro, N.F.P.; Souza, M.M.V.M. Partial oxidation of methane over Ni–Co perovskite catalysts. Catal. Commun. 2011, 12, 665–668. [Google Scholar] [CrossRef]
- Ghiasi, M.; Delgado-Jaime, M.U.; Malekzadeh, A.; Wang, R.-P.; Miedema, P.S.; Beye, M.; de Groot, F.M.F. Mn and Co charge and spin evolutions in LaMn1–xCoxO3 nanoparticles. J. Phys. Chem. C 2016, 120, 8167–8174. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B Environ. 2013, 134–135, 251–257. [Google Scholar] [CrossRef]
- Pecchi, G.; Campos, C.; Peña, O.; Cadus, L.E. Structural, magnetic and catalytic properties of perovskite-type mixed oxides LaMn1−yCoyO3 (y = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9, 1.0). J. Mol. Catal. A Chem. 2008, 282, 158–166. [Google Scholar] [CrossRef]
- Rakshit, S.; Gopalakrishnan, P.S. Oxygen nonstoichiometry and its effect on the structure of LaNiO3. J. Solid State Chem. 1994, 110, 28–31. [Google Scholar] [CrossRef]
- Zhou, C.; Feng, Z.; Zhang, Y.; Hu, L.; Chen, R.; Shan, B.; Yin, H.; Wang, W.G.; Huang, A. Enhanced catalytic activity for NO oxidation over Ba doped LaCoO3 catalyst. RSC Adv. 2015, 5, 28054–28059. [Google Scholar] [CrossRef]
- Qi, G.; Li, W. Pt-free, LaMnO3 based lean NOx trap catalysts. Catal. Today 2012, 184, 72–77. [Google Scholar] [CrossRef]
- Batiot-Dupeyrat, C.; Valderrama, G.; Meneses, A.; Martinez, F.; Barrault, J.; Tatibouët, J.M. Pulse study of Co2 reforming of methane over LaNiO3. Appl. Catal. A Gen. 2003, 248, 143–151. [Google Scholar] [CrossRef]
- Benard, S.; Retailleau, L.; Gaillard, F.; Vernoux, P.; Giroir-Fendler, A. Supported platinum catalysts for nitrogen oxide sensors. Appl. Catal. B Environ. 2005, 55, 11–21. [Google Scholar] [CrossRef]
- Mulla, S.S.; Chen, N.; Cumaranatunge, L.; Blau, G.E.; Zemlyanov, D.Y.; Delgass, W.N.; Epling, W.S.; Ribeiro, F.H. Reaction of NO and O2 to NO2 on Pt: Kinetics and catalyst deactivation. J. Catal. 2006, 241, 389–399. [Google Scholar] [CrossRef]
- Schmitz, P.J.; Kudla, R.J.; Drews, A.R.; Chen, A.E.; Lowe-Ma, C.K.; McCabe, R.W.; Schneider, W.F.; Goralski, C.T. NO oxidation over supported Pt: Impact of precursor, support, loading, and processing conditions evaluated via high throughput experimentation. Appl. Catal. B Environ. 2006, 67, 246–256. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Wang, Y.; Lyu, Y.-K. Performance and mechanism comparison of manganese oxides at different valence states for catalytic oxidation of NO. Chem. Eng. J. 2019, 361, 1161–1172. [Google Scholar] [CrossRef]
- Ivanova, S.; Senyshyn, A.; Zhecheva, E.; Tenchev, K.; Stoyanova, R.; Fuess, H. Crystal structure, microstructure and reducibility of LaNixCo1−xO3 and LaFexCo1−xO3 perovskites (0 <x ≤ 0.5). J. Solid State Chem. 2010, 183, 940–950. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. JACS 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der größe und der inneren struktur von kolloidteilchen mittels röntgenstrahlen. Nachr. Ges. Wiss. Gött. Math. Phys. Kl. 1918, 1918, 98–100. [Google Scholar]
- Tsakoumis, N.E.; Voronov, A.; Rønning, M.; Beek, W.v.; Borg, Ø.; Rytter, E.; Holmen, A. Fischer–tropsch synthesis: An XAS/XRPD combined in situ study from catalyst activation to deactivation. J. Catal. 2012, 291, 138–148. [Google Scholar] [CrossRef]
- Fogler, H. Elements of Chemical Reaction Engineering; Prentice-Hall of India: New Delhi, India, 2006; p. 92. [Google Scholar]








| Sample | Surface Area (m2/g) | d (nm) |
|---|---|---|
| LaCoO3 | 8 | 28 |
| LaCo0.75Mn0.25O3 | 8 | 24 |
| LaCo0.50Mn0.50O3 | 7 | 18 |
| LaMnO3 | 9 | 21 |
| LaCo0.75Ni0.25O3 | 10 | 22 |
| LaCo0.50Ni0.50O3 | 8 | 17 |
| LaCo0.25Ni0.75O3 | 12 | 8 |
| LaNiO3 | 8 | 13 |
| Sample | XNO (%) | rNO 1 (µmolgcat−1s−1) |
|---|---|---|
| LaCoO3 | 24.9 | 5.14 |
| LaCo0.75Mn0.25O3 | 29.7 | 6.55 |
| LaCo0.50Mn0.50O3 | 15.9 | 2.72 |
| LaMnO3 | 18.0 | 3.27 |
| LaCo0.75Ni0.25O3 | 30.3 | 6.70 |
| LaCo0.50Ni0.50O3 | 23.2 | 4.74 |
| LaCo0.25Ni0.75O3 | 16.4 | 2.86 |
| LaNiO3 | 20.8 | 4.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salman, A.u.R.; Hyrve, S.M.; Regli, S.K.; Zubair, M.; Enger, B.C.; Lødeng, R.; Waller, D.; Rønning, M. Catalytic Oxidation of NO over LaCo1−xBxO3 (B = Mn, Ni) Perovskites for Nitric Acid Production. Catalysts 2019, 9, 429. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9050429
Salman AuR, Hyrve SM, Regli SK, Zubair M, Enger BC, Lødeng R, Waller D, Rønning M. Catalytic Oxidation of NO over LaCo1−xBxO3 (B = Mn, Ni) Perovskites for Nitric Acid Production. Catalysts. 2019; 9(5):429. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9050429
Chicago/Turabian StyleSalman, Ata ul Rauf, Signe Marit Hyrve, Samuel Konrad Regli, Muhammad Zubair, Bjørn Christian Enger, Rune Lødeng, David Waller, and Magnus Rønning. 2019. "Catalytic Oxidation of NO over LaCo1−xBxO3 (B = Mn, Ni) Perovskites for Nitric Acid Production" Catalysts 9, no. 5: 429. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9050429