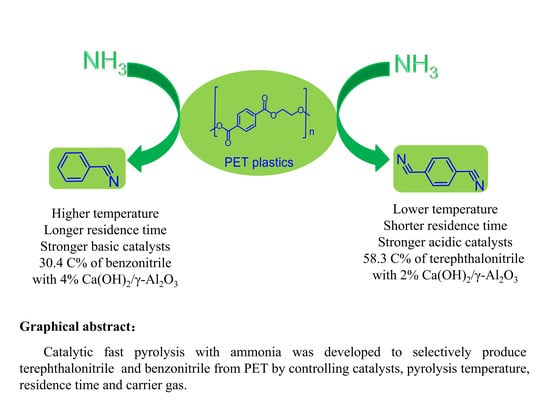
Selective Production of Terephthalonitrile and Benzonitrile via Pyrolysis of Polyethylene Terephthalate (PET) with Ammonia over Ca(OH)2/Al2O3 Catalysts
Abstract
:1. Introduction
2. Results and Discussions
2.1. Effect of Pyrolysis Temperature
2.2. Optimizing Conditions for Producing Terephthalonitrile at 500 °C
2.2.1. Effect of Catalyst
2.2.2. Effect of Residence Time Between the Pyrolytic Vapors and Catalyst
2.2.3. Effect of Ammonia Content in the Carrier Gas
2.3. Optimizing Benzonitrile Production at 650 °C
2.3.1. Effect of Catalyst
2.3.2. Effect of Residence Time
2.3.3. Effect of Ammonia Content
2.4. Possible Reaction Pathways from PET to Terephthalonitrile and Benzonitrile
2.5. Catalysts Stability
2.6. Catalysts Characterzation
3. Materials and Methods
3.1. Materials
3.2. Catalysts Preparation and Regeneration
3.4. Pyrolysis Experiments
3.5. Products Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Halden, R.U. Plastics and Health Risks. Ann. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.; Lee, Y.; Park, S. Optimization of the pre-polymerization step of polyethylene terephthalate (PET) production in a semi-batch reactor. Chem. Eng. J. 1999, 75, 47–55. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Yehia, F.Z.; Harding, D.R.K.; Eshaq, G.; ElMetwally, A.E. Fe3O4-boosted MWCNT as an efficient sustainable catalyst for PET glycolysis. Green Chem. 2016, 18, 3997–4003. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Elordi, G.; Olazar, M.; Bilbao, J. Operating conditions for the pyrolysis of poly-(ethylene terephthalate) in a conical spouted-bed reactor. Ind. Eng. Chem. Res. 2010, 49, 2064–2069. [Google Scholar] [CrossRef]
- Blackmon, K.P.; Fox, D.W.; Shafer, S.J. Process for Converting Pet Scrap to Diamine Monomers. U.S. Patent 4,973,746,11, 1990. [Google Scholar]
- Datta, J.; Kopczyńska, P. From polymer waste to potential main industrial products: Actual state of recycling and recovering. Crit. Rev. Environ. Sci. Technol. 2016, 46, 905–946. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Zhou, G.; Feng, Y.; Wang, Y.; Yu, G. Enhancing the production of renewable petrochemicals by co-feeding of biomass with plastics in catalytic fast pyrolysis with ZSM-5 zeolites. Appl. Catal. A Gen. 2014, 481, 173–182. [Google Scholar] [CrossRef]
- Carlson, T.R.; Cheng, Y.T.; Jae, J.; Huber, G.W. Production of green aromatics and olefins by catalytic fast pyrolysis of wood sawdust. Energy Environ. Sci. 2010, 4, 145–161. [Google Scholar] [CrossRef]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: a review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Conver. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2017, 73, 346–368. [Google Scholar] [CrossRef]
- Yoshioka, T.; Handa, T.; Grause, G.; Lei, Z.; Inomata, H.; Mizoguchi, T. Effects of metal oxides on the pyrolysis of poly (ethylene terephthalate). J. Anal. Appl. Pyrolysis 2005, 73, 139–144. [Google Scholar] [CrossRef]
- Kumagai, S.; Yamasaki, R.; Kameda, T.; Saito, Y.; Watanabe, A.; Watanabe, C.; Teramae, N.; Yoshiok, T. Tandem μ -reactor-GC/MS for online monitoring of aromatic hydrocarbon production via CaO-catalysed PET pyrolysis. React. Chem. Eng. 2018, 2, 776–784. [Google Scholar] [CrossRef]
- Kumagai, S.; Yamasaki, R.; Kameda, T.; Saito, Y.; Watanabe, A.; Watanabe, C.; Teramae, N.; Yoshiok, T. Aromatic hydrocarbon selectivity as a function of CaO basicity and aging during CaO-catalyzed PET pyrolysis using tandem µ-reactor-GC/MS. Chem. Eng. J. 2018, 332, 169–173. [Google Scholar] [CrossRef]
- Du, S.; Valla, J.A.; Parnas, R.S.; Bollas, G.M. Conversion of polyethylene terephthalate based waste carpet to benzene-rich oils through thermal, catalytic, and catalytic steam pyrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2852–2860. [Google Scholar] [CrossRef]
- Xue, Y.; Johnston, P.; Bai, X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Conver. Manag. 2017, 142, 441–451. [Google Scholar] [CrossRef]
- Diaz-Silvarrey, L.S.; McMahon, A.; Phan, A.N. Benzoic acid recovery via waste poly(ethylene terephthalate) (PET) catalytic pyrolysis using sulphated zirconia catalyst. J. Anal. Appl. Pyrolysis 2018, 134, 621–631. [Google Scholar] [CrossRef]
- Xu, L.; Han, Z.; Yao, Q.; Ding, J.; Zhang, Y.; Fu, Y.; Guo, Q. Towards the sustainable production of pyridines via thermo-catalytic conversion of glycerol with ammonia over zeolite catalysts. Green Chem. 2015, 17, 2426–2435. [Google Scholar] [CrossRef]
- Xu, L.; Yao, Q.; Han, Z.; Zhang, Y.; Fu, Y. Producing pyridines via thermocatalytic conversion and ammonization of waste polylactic acid over zeolites. ACS Sustain. Chem. Eng. 2016, 4, 1115–1122. [Google Scholar] [CrossRef]
- Xu, L.; Yao, Q.; Deng, J.; Han, Z.; Zhang, Y.; Fu, Y.; Huber, G.W.; Guo, Q. Renewable N-Heterocycles Production by Thermocatalytic Conversion and Ammonization of Biomass over ZSM-5. ACS Sustain. Chem. Eng. 2015, 3, 2890–2899. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Yang, H.; Li, K.; Chen, X.; Chen, H. Investigation on biomass nitrogen-enriched pyrolysis: Influence of temperature. Bioresour. Technol. 2018, 249, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yao, Q.; Zhang, Y.; Fu, Y. Integrated Production of Aromatic Amines and N-Doped Carbon from Lignin via ex Situ Catalytic Fast Pyrolysis in the Presence of Ammonia over Zeolites. ACS Sustain. Chem. Eng. 2017, 5, 2960–2969. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Z.; Hu, B.; Deng, J.; Yao, Q.; Zhang, X.; Liu, X.; Fu, Y.; Lu, Q. Direct conversion of cellulose and raw biomass to acetonitrile by catalytic fast pyrolysis in ammonia. Green Chem. 2019, 21, 812–820. [Google Scholar] [CrossRef]
- Bornschein, C.; Werkmeister, S.; Wendt, B.; Jiao, H.; Alberico, E.; Baumann, W.; Junge, H.; Junge, K.; Beller, M. Mild and selective hydrogenation of aromatic and aliphatic (di)nitriles with a well-defined iron pincer complex. Nature Commun. 2014, 5, 4111. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, M.S.; Beck, T.M. The Chemistry of Organic Gold Compounds. V. Auration of Aromatic Nitriles. J. Am. Chem. Soc. 2002, 56, 2057–2060. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.J.; Chang, S. Synthesis of Aromatic Nitriles Using Nonmetallic Cyano-Group Sources. Angew. Chem. Int. Ed. 2012, 51, 11948–11959. [Google Scholar] [CrossRef]
- Kholkhoev, B.C.; Burdukovskii, V.F.; Mognonov, D.M. Synthesis of polyamidines based on 1, 4-dicyanobenzene and 4, 4´-diaminodiphenyl oxide in ionic liquids. Russ. Chem. Bull. 2010, 59, 2159–2160. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nagasawa, T.; Yamada, H. Regiospecific hydrolysis of dinitrile compounds by nitrilase from Rhodococcus rhodochrous J1. Appl. Microbial. Biotechnol. 1988, 29, 231–233. [Google Scholar] [CrossRef]
- Hao, L.; Luo, B.; Li, X.; Jin, M.; Fang, Y.; Tang, Z.; Jia, Y.; Liang, M.; Thomas, A.; Yang, J.; Zhi, L. Terephthalonitrile-derived nitrogen-rich networks for high performance supercapacitors. Energy Environ. Sci. 2012, 5, 9747–9751. [Google Scholar] [CrossRef]
- Abuorabi, S.T.; Jibril, I.; Obiedat, R.; Hatamleh, L. Cycloaddition reactions of 2,4,6-trimethoxybenzonitrile oxide with disubstituted acetylenes. 3. J. Chem. Eng. Data 1988, 33, 540–541. [Google Scholar] [CrossRef]
- Zhao, X.; Xiao, H.; Yue, C.Y.; He, Y.; Chen, H. Preparation, characterization and femtosecond time-resolved optical kerr effect of plasma polybenzonitrile derivative films. J. Mater. Sci. Mater. Electron. 2001, 12, 557–560. [Google Scholar] [CrossRef]
- Qiang, F.; Ding, X.; Wu, X.; Yi, Y.; Jiang, L. Synthesis and characterization of novel aromatic ether nitrile monomer containing propenylphenoxy groups and properties of its copolymer with 4,4’-bismaleimidodiphenylmethane. J. Appl. Polym. Sci. 2010, 83, 1465–1472. [Google Scholar]
- Cui, D.; Nishiura, M.; Hou, Z. Lanthanide-imido complexes and their reactions with benzonitrile. Angew. Chem. Int. Ed. 2010, 117, 981–984. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, J. N-heterocyclic carbene-catalyzed convenient benzonitrile assembly. Org. Lett. 2016, 18, 2212–2215. [Google Scholar] [CrossRef]
- Den Ridder, J.J.J.; Van Ingen, H.W.T.J.; van den Berg, P.J. Kinetics of the gas-phase ammoxidation of toluene to benzonitrile on a Bi-Mo-O catalyst. Rec. J. R. Netherland Chem. Soc. 2015, 98, 289–293. [Google Scholar] [CrossRef]
- Narayana, K.V.; Martin, A.; Bentrup, U.; Lücke, B.; Sans, J. Catalytic gas phase ammoxidation of o-xylene. Appl. Catal. A Gen. 2004, 270, 57–64. [Google Scholar] [CrossRef]
- Dumitriu, E.; Hulea, V.; Kaliaguine, S.; Huang, M.M. Transalkylation of the alkylaromatic hydrocarbons in the presence of ultrastable zeolites transalkylation of toluene with trimethylbenzenes. Appl. Catal. A Gen. 1996, 135, 57–81. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Song, H.; Dong, Q.; Dong, G.; Kong, X.; Fang, Z. Catalytic Fast Pyrolysis of Polyethylene Terephthalate Plastic for the Selective Production of Terephthalonitrile under Ammonia Atmosphere. Waste Mang. 2019. [Google Scholar] [CrossRef]






| Entry | Catalyst Dosage (g) | Residence Time (s) | Nitriles (C%) | Aromatics2 (C%) | Nitriles Selectivity (%) | ||
|---|---|---|---|---|---|---|---|
| Terephthalonitrile | Benzonitrile | Other Nitriles3 | |||||
| 1 | 0.6 | 0.94 | 57.05 | 1.44 | 90.48 | 6.60 | 2.52 |
| 2 | 0.8 | 1.25 | 60.53 | 1.51 | 89.91 | 6.81 | 3.29 |
| 3 | 1 | 1.56 | 53.98 | 1.78 | 88.88 | 8.43 | 2.69 |
| 4 | 1.2 | 1.87 | 54.96 | 3.83 | 86.43 | 8.26 | 5.31 |
| 5 | 1.5 | 2.34 | 54.52 | 4.69 | 82.89 | 11.48 | 5.63 |
| Entry | Ammonia Content (%) | Nitriles (C%) | Aromatics2 (C%) | Nitriles Selectivity (%) | ||
|---|---|---|---|---|---|---|
| Terephthalonitrile | Benzonitrile | Other Nitriles3 | ||||
| 1 | 25% | 55.93 | 2.08 | 88.78 | 7.34 | 3.88 |
| 2 | 50% | 60.53 | 1.51 | 89.90 | 6.81 | 3.29 |
| 3 | 75% | 62.93 | 1.49 | 89.51 | 6.63 | 3.86 |
| 4 | 100% | 63.18 | 1.73 | 92.28 | 4.11 | 3.61 |
| Entry | Catalyst Dosage (g) | Residence Time (s) | Nitriles (C%) | Aromatics2 (C%) | Nitriles Selectivity (%) | ||
|---|---|---|---|---|---|---|---|
| Terephthalonitrile | Benzonitrile | Other Nitriles3 | |||||
| 1 | 0.6 | 0.94 | 32.93 | 6.33 | 28.36 | 64.74 | 6.89 |
| 2 | 0.8 | 1.25 | 31.14 | 7.85 | 15.35 | 77.91 | 6.74 |
| 3 | 1 | 1.56 | 33.1 | 10.11 | 12.36 | 81.12 | 6.53 |
| 4 | 1.2 | 1.87 | 32.96 | 13.94 | 6.43 | 84.98 | 8.59 |
| 5 | 1.5 | 2.34 | 32.05 | 15.87 | 4.21 | 84.21 | 11.58 |
| Entry | Ammonia Content (%) | Nitriles (C%) | Aromatics2 (C%) | Nitriles Selectivity (%) | ||
|---|---|---|---|---|---|---|
| Terephthalonitrile | Benzonitrile | Other Nitriles3 | ||||
| 1 | 25% | 25.53 | 15.81 | 7.46 | 84.04 | 8.5 |
| 2 | 50% | 32.96 | 13.94 | 6.43 | 84.98 | 8.59 |
| 3 | 75% | 34.14 | 13.5 | 6.85 | 84.33 | 8.82 |
| 4 | 100% | 36.83 | 12.56 | 8.12 | 82.60 | 9.28 |
| Entry | Feedstock | Nitriles (C%) | Aromatics 2 (C%) | Nitriles Selectivity (%) | ||
|---|---|---|---|---|---|---|
| Terephthalonitrile | Benzonitrile | Other Nitriles3 | ||||
| 1 | Benzoic acid | 68.75 | 3.02 | N.D | 100 | N.D |
| 2 | Methyl benzoate | 64.34 | 3.46 | N.D | 98.78 | 1.22 |
| 3 | Benzamide | 86.56 | 5.68 | N.D | 100 | N.D |
| 4 | Terephthalic acid | 56.05 | 2.89 | 53.02 | 45.43 | 1.55 |
| 5 | Dimethyl terephthalate | 59.52 | 3.27 | 49.59 | 47.54 | 2.86 |
| 6 | Benzonitrile | 95.74 | 0.45 | N.D | 100 | N.D |
| Entry | Catalysts | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Total Acid Amounts (μmol NH3/g) | Total Basic Amounts (μmol CO2/g) |
|---|---|---|---|---|---|
| 1 | 2% Ca(OH)2/γ-Al2O3 | 148.14 | 0.70 | 303.91 | 197.54 |
| 2 | 4% Ca(OH)2/γ-Al2O3 | 147.13 | 0.69 | 377.05 | 241.24 |
| 3 | Used 2% Ca(OH)2/Al2O3 1 | 116.76 | 0.56 | 295.74 | 77.40 |
| 4 | Used 4% Ca(OH)2/Al2O3 2 | 122.63 | 0.60 | 160.58 | 99.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Na, X.-w.; Zhang, L.-y.; Dong, Q.; Dong, G.-h.; Wang, Y.-t.; Fang, Z. Selective Production of Terephthalonitrile and Benzonitrile via Pyrolysis of Polyethylene Terephthalate (PET) with Ammonia over Ca(OH)2/Al2O3 Catalysts. Catalysts 2019, 9, 436. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9050436
Xu L, Na X-w, Zhang L-y, Dong Q, Dong G-h, Wang Y-t, Fang Z. Selective Production of Terephthalonitrile and Benzonitrile via Pyrolysis of Polyethylene Terephthalate (PET) with Ammonia over Ca(OH)2/Al2O3 Catalysts. Catalysts. 2019; 9(5):436. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9050436
Chicago/Turabian StyleXu, Lujiang, Xin-wen Na, Le-yao Zhang, Qian Dong, Guo-hua Dong, Yi-tong Wang, and Zhen Fang. 2019. "Selective Production of Terephthalonitrile and Benzonitrile via Pyrolysis of Polyethylene Terephthalate (PET) with Ammonia over Ca(OH)2/Al2O3 Catalysts" Catalysts 9, no. 5: 436. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9050436