1. Introduction
Natural gas is a clean fossil fuel, and the global consumption of natural gas has increased steadily in the last decade [
1,
2,
3,
4,
5,
6]. Nevertheless, reserves of natural gas are expected to be depleted in the next few decades and its prices are continuously rising [
7,
8,
9,
10]. In this respect, synthetic natural gas (SNG) production from syngas from coal or renewable biomass has received significant attention in some countries. The conventional route for SNG procedure is in accordance with the gasification and methanation of coal to synthetic gas (CO+H
2) [
4,
11,
12]. More recently, at least 15 coal-to-gas plants are planned in the United States, and more than 20 coal-to-gas plants are under construction and planned in China [
13]. CO reaction (CO + 3H
2 → CH
4 + H
2O, ∆
H298K = −206.1 kJ mol
−1), as one of the fundamental steps in making coal-formed gas process, has raised broad interest after Sabatier and Senderens originally discovered it in 1902 [
14]. This can to a large extent be attributed to its broad utility in chemical production, e.g. elimination of micro amounts of CO from H
2-rich feed gas, purification of reforming gas for fuel-cell manufacture, technologies in respect of Fischer–Tropsch synthesis production, among others [
15,
16,
17,
18]. Hence, exploiting a new and high-efficiency catalyst for methanation is nowadays a matter of great concern.
Normally, Ni supported on Al
2O
3 is one of the most widely studied catalysts in methanation reactions for the production of SNG as a result of its lower price, higher catalytic activity, and the better selectivity for the methane product [
19,
20,
21,
22,
23]. Al
2O
3, especially the γ-modification with larger specific surface area and mechanical strength, developed pore structure, and greater acid and alkali resistance on the surface, strongly affects the catalytic performance, which increases activity and stability for methanation [
24,
25]. However, methanation, as a key step of SNG production, is a highly exothermic reaction. For each 1% CO and each 1% CO
2, the representative methanation gas component temperature rise in ammonia plants is 74 and 60 °C, separately [
26]. In the high-temperature reaction environment, the Ni-based methanation catalyst is prone to sintering, resulting in the accelerated migration and aggregation of the nickel microcrystalline particles, and the decrease of the nickel dispersion and the effective specific surface area [
27,
28]. Al
2O
3 support also suffers the disadvantage of poor thermal stability. Thus, the catalytic activity is reduced. Therefore, solving the catalyst sintering problem is the biggest challenge for the methanation process.
Many efforts have been devoted to further increasing the performance of resistance to sintering of Ni/Al
2O
3 catalyst. The sintering rate of the catalyst is related to the size and distribution of the nickel crystallites, the structure, morphology and transition state of the support [
26]. Adding additives is one of the most effective means to improve the anti-sintering performance of the catalyst. For example, adding ZrO
2 can sequester NiO and γ-Al
2O
3 and weaken their interactions, which play the role of limiting the aggregation of γ-Al
2O
3 [
17]. The addition of Rh and Ru was reported to improve the heat stability of nickel loading on alumina [
29,
30]. Adding La
2O
3 [
1], MgO [
31] can form LaAlO
3 and MgAl
2O
4 surface layers and prevent their activity component Ni
2+ diffused into the Al
2O
3 bulk phase to form NiAl
2O
4, slowing down the catalyst sintering rate. CeO
2 was added to inhibit the migration of Ni atoms on the surface of Al
2O
3 and improve the dispersion of nickel particles owing to the strong metal–support interaction (SMSI) [
32]. Recently, Ma et al [
33] and Bao and coworkers [
34] reported that encapsulating Ni particles with graphene or hexagonal boron nitride shells could enhance the anti-sintering ability of Ni-based methanation catalysts.
Although a lot of research efforts have been made towards searching for new promoters to improve the resistance to sintering of Ni/Al
2O
3 catalysts for methanation [
31,
32,
33,
34,
35,
36,
37], it is difficult to understand the intrinsic contribution of various promoters because of the complexity of the catalytic mechanism. As a result, the exploration of new additives for methanation catalysts has mainly been dependent on trial and error methods until now.
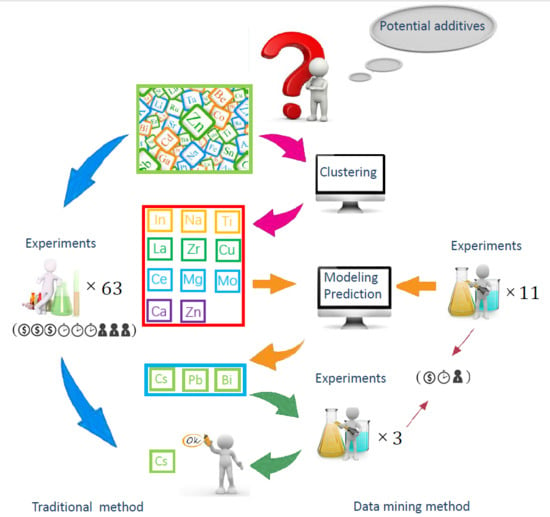
In contrast with the traditional catalyst development, whereby researchers obtain all the handled experimental results and provide guidance for the subsequent studies, data mining as a new approach does not require any starting hypotheses, since it can be readily applied to any non-linear catalytic phenomena and global search potential correlations and modes between the input variables (catalysts component, preparation conditions, and reaction parameters) and output variables (catalysts characterization and performance) through massive amounts of raw information [
38]. In our previous work, data mining techniques was used to screen potential additives of Ni/Al
2O
3 catalyst for improving the catalytic activity [
39]. We constructed a regression model through data mining and it forecast that as a potential auxiliary agent, Re improves the catalytic activity. There is an acceptable agreement between the predicted and experimental results. More specifically, it demonstrated that Re can be considered to be the most effective of all additives. These powerful computational techniques avoid the blind search process and complex interactions within the catalyst and could expedite the design of new catalyst systems.
Anti-sintering performance is another important indicator of methanation catalysts [
40,
41]. It is of great significance to predict and screen new additives based on existing experimental data. And as such the aim was to select element additives, which can retain high resistance to sintering at long-term high temperatures. In this paper, data mining was used to build an optimization model and to forecast the resistance to sintering of Ni/Al
2O
3 catalysts with different additives, thus screening the optimal additives.
2. Results and Discussion
Data mining is an emerging concept, mainly referring to the process of knowledge discovery in databases. It constantly explores how to extract knowledge that is difficult to obtain through theoretical calculations from some massive and seemingly disorganized observation data. This knowledge often has the following characteristics: it is previously unknown, effective and practical. It is then used to analyze practical problems and make effective predictions for complex, fuzzy, and jagged datasets. Normal process of the data mining is shown in
Figure 1. The general procedure of the data mining is summarized, including a) data collection, b) data cleaning, c) data transformation, d) data loading, e) data analysis and f) result report. Owing to the diversity of data sources, aggregation is required prior to loading the data [
41]. The specific explanation for using data-mining screen additives of Ni/Al
2O
3 catalyst is as follows:
2.1. Data Collection
This design aims to model the relationship between the additives of Ni/Al
2O
3 catalyst and its anti-sintering performance through data-mining technology. The optimal additive is then predicted and screened. Therefore, when modeling, different additive elements are taken as input, and their corresponding anti-sintering properties are taken as output. The basic process and data to be collected is shown in
Figure 2.
2.1.1. Input Data
Based on previous research results, 63 elements except for gases, poisons, radioactive ones were selected as candidate additives for Ni/Al
2O
3 catalyst. The inter-relationship between the type of the element additives and the resistance to sintering of Ni/Al
2O
3 catalysts is then established. Here, the additive element cannot be applied straightforwardly as input variables and need to be transformed into a series of representative data. Physicochemical properties of elements affect the catalytic performance, i.e. specific surface area, metal dispersion degree, electron configuration, morphology features, and heat stability. These properties, in sequence, identify the catalytic performance [
42,
43,
44,
45]. Moreover, the physicochemical properties of additive elements were selected as explanatory variables that describe each element and were used for the prediction of the catalytic performance in the process of building the regression model.
In this study, 16 physicochemical properties were utilized as proper descriptors for each element, illustrated by
Table 1. In the field of data-mining technology, these properties were recorded in the “data.csv” file, as explained in
Table 2, where only a portion of the file is displayed. The properties are stored in the 1–16th columns. Correlated symbols are stored in the 17th column, simultaneously.
16 physicochemical properties as the explanatory variables were reduced by principal component analysis (PCA), which is clarified in
Section 2.3.
2.1.2. Output Data
For the purpose of ensuring the accuracy of regression model, data of 63 elements that enable the whole periodic table to be represented were available for K-means element cluster analysis [
46,
47]. Subsequently, one or two characteristic elements were chosen for each cluster. A pool of 11 elements (Na, Ca, Ce, Mg, La, Cu, Zn, Zr, In, Mo, and Ti) was chosen for the corresponding experiment.
The obtained experimental data were shown in
Table 3. Bare is the Ni/Al
2O
3 catalyst. The crystallite sizes of the Ni, before and after catalyst evaluation, were marked as d and d’. The variation of crystallite sizes of the Ni were marked as d
△.
Sintering leads to an increase in Ni crystallites, which is one of the main reasons for catalyst deactivation. The increase rate of crystallite size of the Ni was selected as output variables and used for data analysis. The lower the increase rate, the better anti-sintering performance.
2.2. Data Cleaning
Data cleaning can be defined as a process of which damages or errors are detected and corrected (or eliminated) in data representation among data sources. The ultimate goal is to gain high-quality data that is elementary for accurate data analysis [
48]. The datasets assembled in our work is intact and reliable, and there is no need to clean.
2.3. Data Transformation
The use of 16 physicochemical properties to characterize each additive element means that the input variable is transformed into a set of 16-dimensional data. At this point, the input variable has a larger dimension. High dimensionality makes analysis extremely difficult and unfavorable for modeling; additionally, the required computing time would become much longer [
49]. Hence, reducing the amount of input variables is crucial through data transformation.
Dimension reduction is of great importance; on the one hand, high-dimensional data cannot be directly applied in some specific algorithm, dimension reduction can solve the “dimension disaster” (that is involved in vector calculation problems, computation exponentially multiply with the increase of digits), reduce the complexity of data, ensuring some algorithms can be used normally; On the other hand, high-dimensional data often contain a lot of noise and redundancy. Dimensional reduction can refine the data structures of interest in high-dimensional data and present them in low-dimensional space in order to better understand the data.
PCA is used in almost all scientific disciplines in reducing dimensionality [
50]. PCA is based on the location distribution of the sample point in the multidimensional pattern space, the maximum direction of variance, which is the maximum direction of the change of the sample point in the space, as the discriminant vector to realize the feature extraction and data compression of the data. From the point of view of probability and statistics, the greater the variance of a random variable, the more information the random variable contains. If the variance of a variable is zero, this variable is a constant value and it does not contain any information. The so-called principal components are new variables obtained by a linear combination (or mapping) of several variables of the original data. The first principal component might have the maximum variance; each principal component is linearly independent, that is, orthogonal. From the first principal component, the principal components are arranged in the order of variance (i.e. the corresponding eigenvalues are arranged in the order of size). The later principal components are considered to be included in the noise and redundancy, and these variables are not introduced into the model in the analysis. Thus PCA can conduce to extracting most of the signal into the first few principal components, reducing the principal component of the analysis to achieve the purpose of reducing the dimension.
The number of input variables (16 physicochemical properties) was lessened by means of PCA. As can be seen in
Figure 3, the cumulative contribution ratio versus the amount of principal component is presented.
The scree plot indicates that the cumulative contribution radio of 6 principal components involves more than 85% information. As a matter of fact, 6 principal components are adequate to generalize the characteristic of 16 physicochemical properties. Thereby, 16 physicochemical properties were converted to 6 principal components, which were used for data mining. The details were recorded in the “pcdata.csv” file as shown in
Table 4.
2.4. Data Loading
Data mining (
Supplementary Materials) was conducted in our work using the statistic language R. The datasets were loaded on R and the main functions used were:
2.5. Data Analysis
As mentioned earlier, the models for screening potential additives was determined using the 22 data sets comprised the input data, that is, six principal components of 11 elements, and the target values, containing resistance to sintering feature. However, a small sample of data makes processing and modeling tough. Prior modeling with small data sets, an adaptable data analysis methodology was chosen properly to overcome this limitation.
The successful application of various widespread modeling and optimizing approaches has been already investigated and reported by many researchers in the design of catalysts [
53,
54,
55,
56,
57]. So far, artificial neural networks (ANN) and support vector machines (SVM) are the most universal because of their wide range of suitability, particularly in nonlinear catalytic phenomena for finding correlations. However, the accuracy of ANN and SVM methods utilized for modeling and predicting was not sufficient in this research with respect to the viewpoint of regression models.
As original methods, ANN and SVM are suitable for processing large datasets; the model should be therefore constructed using a mass of validation data to ensure its applicability and reliability. Considering the situation above, those two methods are not appropriate for data analysis of small sample datasets.
Input of several key parameters utilized frequently in ANN and SVM can have a large impact on the feasibility of the model; for instance, the type of kernel functions adopted by an SVM, or the structure design parameter of a neural network. Parameter optimisation is significant and become more difficult on the basis of small sample datasets.
There are no indicators in ANN and SVM for further improving accuracy of the regression model.
To tackle these obstacles, a Gaussian process regression (GPR) methodology was applied to promote the catalytic performance of the resistance to sintering by establishing a regression model in accordance with the physicochemical properties of each selected element. The major advantage for exploiting this probabilistic nonparametric modeling method into the resistance to sintering of the catalysts optimization is that, accuracy of the modeling prediction and applicability of analyzing high-dimensional, nonlinear, small sample datasets [
58,
59].
where
is the weight,
the center vector, and
the radius. Thus, the output
is calculated from the input vector
.
It is difficult to find appropriate parameters when modeling with small datasets. In the present study, a nonparametric algorithm is applied to the GPR model, that is, the estimated parameters are not necessary. The weights of GPR, as an a posteriori probability, were determined. Moreover, a unique radius parameter in each dimension was employed in the bgp function. Both improvements made for better performance of the GPR.
In particular, expected improvement (EI) function was introduced into the GPR and the indicator EI was produced [
60]. EI that serves as discrimination criteria of the new potential optimal solution manifests itself in possibly increasing the predictability and feasibility of the GPR model since the experimental results were applied to the modeling. Therefore, imperative supplementary tests are forecast by the index EI. If the largest EI is approaching zero, then the optimization process can be completed owing to no further expected improvement which is found by performing supplementary tests.
Figure 4 displays a flowsheet for the process of modeling and anticipating by data mining techniques in this part of work.
2.6. Result Report
For developing the GPR model, as in
Table 4, model input data were PC1–PC6 for Na through Ti. Meanwhile, the increase rate of Na through Ti has been utilized as model outputs, summarized in
Table 3. Thereafter,
Table 4 clearly gives PC1–PC6 for Cs through Re, used for predicting the outcome in GPR model.
Figure 5 respectively illustrates the increase rate of Cs through Re, which has been already predicted by aforementioned procedure, and ranked elements in accordance with the EI.
From the perspective of additional experiments, effectively additive elements of Pb, Cs, and Bi were explored, according to their high resistance to sintering of the catalysts and high EI. The predicted increased rate (
Means) and the 90% confidence interval (
q1,
q2) are listed in
Table 5. Thereby, experiments with respect to the anti-sintering of Pb, Cs, and Bi, which promoted Ni/Al
2O
3 catalytic performance, were executed. More detailed data correlated with resistance to sintering are revealed in
Table 6 and experimentally determined.
According to these results, it is apparent that three additive elements, namely, the Pb, Cs, and Bi, with resistance to sintering superior than one of Ni-In/Al
2O
3 catalysts, which were estimated to be candidates. However, this prediction was incorrect. The increase rate for Ni-Pb/Al
2O
3, Ni-Cs/Al
2O
3, and Ni-Bi/Al
2O
3 catalysts are higher than that of Ni-In/Al
2O
3 catalysts. Thereafter, additional experiments were carried out utilizing Pb, Cs, as well as Bi element for building the second regression model by GPR. In this context, as depicted by
Figure 6, elements were ranked with respect to the EI. Through the prediction of the second regression model, it is found that among the remaining elements, adding Li has the best anti-sintering performance, and the detailed data are shown in
Table 7. However, the experimental results reveal that the effect of adding Li is not as good as that of adding Cs; furthermore, its EI value is quite low. Consequently, the screening process was eventually finished.
It is noticeable that, as one of the Ni/Al2O3 catalytic promoters, Cs addition possessed exceptional performance, which was determined by the predicted model outputs and its corresponding experimental values. Although the increase rate of Ni-Cs/Al2O3 catalysts are higher than that of Ni-In/Al2O3 catalysts, its crystallite sizes of the Ni, before and after catalyst evaluation, are less than those of Ni-In/Al2O3 catalysts. Apart from those mentioned above, more significantly, it has the minimal variation of crystallite sizes of the Ni.
2.7. Effect of Physicochemical Properties on Catalytic Anti-Sintering
The superiority of regression model by GPR in finding an anti-sintering Ni-Cs/Al2O3 catalyst is verified by the obtained results. It obviously shows that the proposed model can quickly search for potential optima with respect to improvement of regression accuracy and suggestion of necessary additional experiments. Another major concern is whether underlying non-linear correlations or patterns are existed in the regression model, which is determined by GPR, between the physicochemical properties and catalytic performance of resistance to sintering. Based on the method of virtual elements, the effect of physicochemical properties of each element on resistance to sintering was calculated.
As indicated in
Table 2, one of the physicochemical properties of Cs was selected to change from the minimum to the maximum of the performance of 63 elements. Accordingly, the six principal components and the growth rate predicted by GPR model were also changed. Consequently, the effect of the physicochemical property can be estimated. Difference of the predicted increased rate chosen here, that is, corresponding to the difference between 20% and 100% of the normalized physicochemical property, represented the influence of different physicochemical properties on the anti-sintering performance of the catalyst, by reason that the effects were monotonous. As can be seen in
Figure 7, the effects of size factors, for instance, IR (ionic radii), DS (density), and AW (atomic weight) are large in the sintering resistance, while the effects of thermal properties such as HV (heat of vaporization), MP (melting point), BP (boiling point) are small. Particularly, IR (ionic radii) is an important factor to limit Ni sintering. The difference of the predicted increased rate obtained by calculation, whose absolute value greater than 0.6, is the largest of all values. In this manner, the influence of each property on the catalytic anti-sintering clearly showed that some properties are influential and others are not. Whereas how those different properties affect the anti-sintering performance is not revealed immediately, this should be clarified in further research.
3. Experimental Section
3.1. Materials
All the reagents were of analytical grade and used as received, which are embodied below: NaNO3, Ca(NO3)2·4H2O, Ce(NO3)3·6H2O, Mg(NO3)2·6H2O, La(NO3)3·6H2O, Cu(NO3)2·3H2O, Zr(NO3)4·5H2O, Zn(NO3)2·6H2O, In(NO3)3·4.5H2O, H24Mo7N6O24·4H2O, C16H36O4Ti, Cs2CO3, Bi(NO3)3·5H2O, PbC4H6O4·3H2O, Ni(NO3)2·6H2O, and deionized water. After calcining in air at 550 °C for 6 h, commercial γ-Al2O3 can be used.
3.2. Catalyst Preparation
Presently, a total of fourteen elements (Na, Ca, Ce, Mg, La, Cu, Zn, Zr, In, Mo, Ti, Cs, Bi, and Pb) were selected as candidates in the experiment for the additives to supported Ni/Al
2O
3 catalyst. And the justification for choosing these elements is illustrated in the
Section 2.1. A successive impregnation method, as described in our pervious paper, was implemented to synthesize the catalysts [
39].
To result into the Ni/Al2O3 catalyst with 15 wt% Ni, this typical synthesis procedure was as follows. Initially, the support was added to deionize aqueous solution with appropriate concentration of nickel nitrate hexahydrate with vigorous stirring. The slurry with continuous stirring was then maintained at room temperature overnight. Afterwards, the heating of the solution was controlled at 80 °C until the water completely evaporated. Followed by drying process at 110 °C in air for 12 h, the mixture finally calcined at 550 °C in static air during 4 h at a rate of 3 °C min−1.
The Ni-X/Al2O3 catalysts (X = Na, Ca, Ce, Mg, La, Cu, Zn, Zr, In, Mo, Ti, Pb, Cs, or Bi) with a Ni/X molar ratio of 3 were further obtained utilizing the method mentioned above. Operational details about relevant experimental process are presented by the same authors. Hence, this only gives a brief description of the points that need to be noticed in this process. Two grams of ready-prepared Ni/Al2O3 catalyst were required for making up the corresponding aqueous solution. Thereafter, 1.704 mmol of added nitrate reagent was dissolved in the precursor solution to form a suspension. Lastly, through the same rate of heating, a calcination treatment was conducted in a muffle furnace for 4 h at 450 °C.
3.3. Catalyst Evaluation
The evaluation of the collected catalysts resistance to sintering for CO methanation reaction was performed at 1 MPa pressure in a fixed bed continuous flow reactor, which made of stainless-steel tubing (i.d. 10mm). About 200 mg of powder catalysts (40–80 mesh), which dispread between quartz wool at the center of the reactor, was reduced at 500 °C in a hydrogen flow of 25 mL min−1 for 2 h before starting each experiment and then cooled to the temperature of 240 °C. The resulting mixed gas here comprised H2 and CO with molar ratio of 3 was then fed into the reactor. The weight hourly space velocity (WHSV) was maintained at 30,000 mL g−1 h−1. The temperature range of the measured was between 240 and 800 °C. The observation points selected are spaced at an interval of 40 °C. To evaluate the sintering resistance of the catalyst more effectively, the temperature was kept at 800 °C for 24 h. Meanwhile, the outlet gas composition (H2, CO, CH4 and CO2) was analyzed using an online gas chromatograph instrument, which was equipped with thermal conductivity detector (TCD), condensing and separating the generated water before the analysis.
3.4. Structural Characterization
The conventional Ni-based catalysts for this reaction often deactivate severely due to sintering of the Ni particles, which is caused by the poor dispersion of nickel particles because of the weak metal–support interaction between Ni and support [
61]. In order to gain an insight into the structure–catalytic anti-sintering properties relationship, powder X-ray diffraction (XRD) characterizations of the catalysts before and after the catalytic reaction were performed in the present study [
11].
XRD patterns of the tested samples were obtained in step scanning on a Rigaku D/Max 2500 diffractometer utilizing CuKα radiation (
λ = 1.54056 Å) operating at 40 kV and 100 mA over a 2
θ range of 10-80° at 4° min
−1. The crystallite size characterization of the Ni on the basis of (1, 1, 1) surface was calculated using the following Scherrer–Warren equation [
62,
63]:
where
κ represents Scherrer’s constant, which is 0.94, and
λ (incident wavelength) is 1.5418 Å. The half-height width of diffraction peak of the tested sample is described as
β. Bragg diffraction angle, which represented as parameter of
θ, is need to be measured. Here,
only as the descriptor of the crystalline size of perpendicular to the direction of the grain surface has nothing to do with the other directions.
4. Conclusions
In this paper, data-mining technology were successfully developed to the modeling and screening of methanation catalysts. The data mining technology could be conveniently applied to build a new modeling framework and prediction from the high-dimensional, discrete and complex catalytic data arising from catalysis experimentation. The application of regression models within physicochemical properties of elements utilized as promoters of Ni/Al2O3 catalysts has been implemented to predict the resistance to sintering of Ni/Al2O3 catalysts in the field of methanation reactions. A better catalyst, adding Cs into Ni/Al2O3 catalyst, which was discovered by utilizing the regression model with GPR, could obtain excellent anti-sintering catalytic performance. Furthermore, on account of using virtual elements, the influence of physicochemical properties on the anti-sintering was evaluated.
More importantly, the agreement between predicted and observed experiment values was highly acceptable, therefore demonstrating the viability of data-mining techniques in the analysis and prediction of resistance to sintering. This work confirmed that the novel data mining approach with avoiding the huge and blind experimental process may eventually serve as a scientific and efficient tool to optimize the design and screening of new catalytic systems in a more rapid manner.