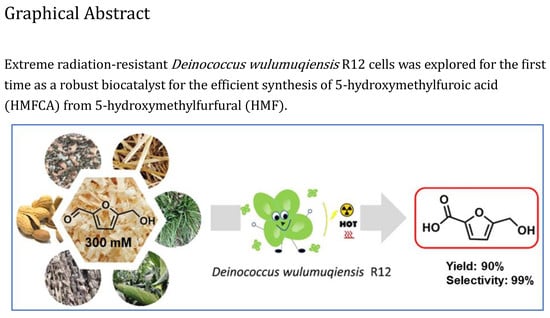
Highly Selective Oxidation of 5-Hydroxymethylfurfural to 5-Hydroxymethyl-2-Furancarboxylic Acid by a Robust Whole-Cell Biocatalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Growing and Resting Deinococcus Cells as Catalysts in HMF Oxidation with Selective Formation of HMFCA
2.2. Effect of Cell Dosage in the Reaction System for HMFCA Synthesis
2.3. Effect of Temperature and pH on HMFCA Synthesis
2.4. Inhibitory and Toxic Effect of Substrate
2.5. Inhibitory and Toxic Effect of the HMFCA Product
2.6. Manufacture of HMFCA Under Optimized Conditions
2.7. Efficient Synthesis of HMFCA by a Fed-Batch Strategy
2.8. Exploring the Substrate Scope of D. wulumuqiensis R12
3. Materials and Methods
3.1. Chemicals and Strains
3.2. Cultivation of D. wulumuqiensis R12 Cells
3.3. General Procedure for the Biocatalytic Oxidation of Aldehyde Substrates
3.4. Analytical Method
3.5. Cell Viability Assay
3.6. Synthesis of HMFCA by the Substrate Fed-Batch Feeding Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Wu, L.; Moteki, T.; Gokhale, A.A.; Flaherty, D.W.; Toste, F.D. Production of Fuels and Chemicals from Biomass: Condensation Reactions and Beyond. Chem 2016, 1, 32–58. [Google Scholar] [CrossRef] [Green Version]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deng, K. Recent Advances in the Catalytic Synthesis of 2,5-Furandicarboxylic Acid and Its Derivatives. ACS Catal. 2015, 5, 6529–6544. [Google Scholar] [CrossRef]
- Christensen, C.H.; Rass-Hansen, J.; Marsden, C.C.; Taarning, E.; Egeblad, K. The Renewable Chemicals Industry. ChemSusChem 2008, 1, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Chheda, J.N.; Román-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2026. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Koopman, F.; Wierckx, N.; de Winde, J.H.; Ruijssenaars, H.J. Efficient whole-cell biotransformation of 5-(hydroxymethyl)furfural into FDCA, 2,5-furandicarboxylic acid. Bioresour. Technol. 2010, 101, 6291–6296. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; He, A.; Liu, X.; Xia, J.; Xu, J.; Zhou, S.; Xu, J. Biocatalytic Transformation of 5-Hydroxymethylfurfural into High-Value Derivatives: Recent Advances and Future Aspects. ACS Sustain. Chem. Eng. 2018, 6, 15915–15935. [Google Scholar] [CrossRef]
- Gandini, A.; Silvestre, A.J.D.; Neto, C.P.; Sousa, A.F.; Gomes, M. The furan counterpart of poly(ethylene terephthalate): An alternative material based on renewable resources. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 295–298. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Hirai, H. Oligomers from Hydroxymethylfurancarboxylic Acid. J. Macromol. Sci. Part A Chem. 1984, 21, 1165–1179. [Google Scholar] [CrossRef]
- Tamura, G. Antitumor Activity of 5-Hydroxy-methyl-2-furoic Acid AU—Munekata, Masanobu. Agric. Biol. Chem. 1981, 45, 2149–2150. [Google Scholar] [CrossRef]
- Braisted, A.C.; Oslob, J.D.; Delano, W.L.; Hyde, J.; McDowell, R.S.; Waal, N.; Yu, C.; Arkin, M.R.; Raimundo, B.C. Discovery of a Potent Small Molecule IL-2 Inhibitor through Fragment Assembly. J. Am. Chem. Soc. 2003, 125, 3714–3715. [Google Scholar] [CrossRef]
- Casanova, O.; Iborra, S.; Corma, A. Biomass into Chemicals: Aerobic Oxidation of 5-Hydroxymethyl-2-furfural into 2,5-Furandicarboxylic Acid with Gold Nanoparticle Catalysts. ChemSusChem 2009, 2, 1138–1144. [Google Scholar] [CrossRef]
- Gorbanev, Y.Y.; Klitgaard, S.K.; Woodley, J.M.; Christensen, C.H.; Riisager, A. Gold-Catalyzed Aerobic Oxidation of 5-Hydroxymethylfurfural in Water at Ambient Temperature. ChemSusChem 2009, 2, 672–675. [Google Scholar] [CrossRef]
- Davis, S.E.; Houk, L.R.; Tamargo, E.C.; Datye, A.K.; Davis, R.J. Oxidation of 5-hydroxymethylfurfural over supported Pt, Pd and Au catalysts. Catal. Today 2011, 160, 55–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, B.; Lv, K.; Sun, J.; Deng, K. Aerobic oxidation of biomass derived 5-hydroxymethylfurfural into 5-hydroxymethyl-2-furancarboxylic acid catalyzed by a montmorillonite K-10 clay immobilized molybdenum acetylacetonate complex. Green Chem. 2014, 16, 2762–2770. [Google Scholar] [CrossRef]
- Zhou, B.; Song, J.; Zhang, Z.; Jiang, Z.; Zhang, P.; Han, B. Highly selective photocatalytic oxidation of biomass-derived chemicals to carboxyl compounds over Au/TiO2. Green Chem. 2017, 19, 1075–1081. [Google Scholar] [CrossRef]
- Subbiah, S.; Simeonov, S.P.; Esperança, J.M.S.S.; Rebelo, L.P.N.; Afonso, C.A.M. Direct transformation of 5-hydroxymethylfurfural to the building blocks 2,5-dihydroxymethylfurfural (DHMF) and 5-hydroxymethyl furanoic acid (HMFA) via Cannizzaro reaction. Green Chem. 2013, 15, 2849–2853. [Google Scholar] [CrossRef]
- Kang, E.-S.; Chae, D.W.; Kim, B.; Kim, Y.G. Efficient preparation of DHMF and HMFA from biomass-derived HMF via a Cannizzaro reaction in ionic liquids. J. Ind. Eng. Chem. 2012, 18, 174–177. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Guajardo, N. Biocatalytic Valorization of Furans: Opportunities for Inherently Unstable Substrates. ChemSusChem 2017, 10, 4123–4134. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zong, M.-H.; Li, N. Whole-cell biocatalytic selective oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid. Green Chem. 2017, 19, 4544–4551. [Google Scholar] [CrossRef]
- Qin, Y.-Z.; Li, Y.-M.; Zong, M.-H.; Wu, H.; Li, N. Enzyme-catalyzed selective oxidation of 5-hydroxymethylfurfural (HMF) and separation of HMF and 2,5-diformylfuran using deep eutectic solvents. Green Chem. 2015, 17, 3718–3722. [Google Scholar] [CrossRef]
- Mitsukura, K.; Sato, Y.; Yoshida, T.; Nagasawa, T. Oxidation of heterocyclic and aromatic aldehydes to the corresponding carboxylic acids by Acetobacter and Serratia strains. Biotechnol. Lett 2004, 26, 1643–1648. [Google Scholar] [CrossRef]
- Van Rantwijk, F.; Sheldon, R.A. Chloroperoxidase-Catalyzed Oxidation of 5-Hydroxymethylfurfural. J. Carbohydr. Chem. 1997, 16, 299–309. [Google Scholar] [CrossRef]
- Krystof, M.; Pérez-Sánchez, M.; Domínguez de María, P. Lipase-Mediated Selective Oxidation of Furfural and 5-Hydroxymethylfurfural. ChemSusChem 2013, 6, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Xu, J.-H. Biocatalytic ketone reduction: A green and efficient access to enantiopure alcohols. Biotechnol. Adv. 2012, 30, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Ku, S. Finding and Producing Probiotic Glycosylases for the Biocatalysis of Ginsenosides: A Mini Review. Molecules 2016, 21, 645. [Google Scholar] [CrossRef] [PubMed]
- Trudgill, P.W. The metabolism of 2-furoic acid by Pseudomanas F2. Biochem. J. 1969, 113, 577–587. [Google Scholar] [CrossRef]
- Koenig, K.; Andreesen, J.R. Xanthine dehydrogenase and 2-furoyl-coenzyme A dehydrogenase from Pseudomonas putida Fu1: Two molybdenum-containing dehydrogenases of novel structural composition. J. Bacteriol. 1990, 172, 5999–6009. [Google Scholar] [CrossRef]
- López, M.J.; Nichols, N.N.; Dien, B.S.; Moreno, J.; Bothast, R.J. Isolation of microorganisms for biological detoxification of lignocellulosic hydrolysates. Appl. Microbiol. Biotechnol. 2004, 64, 125–131. [Google Scholar] [CrossRef]
- Koopman, F.; Wierckx, N.; de Winde, J.H.; Ruijssenaars, H.J. Identification and characterization of the furfural and 5-(hydroxymethyl)furfural degradation pathways of Cupriavidus basilensis HMF14. Proc. Natl. Acad. Sci. USA 2010, 107, 4919–4924. [Google Scholar] [CrossRef]
- Nikel, P.I.; Chavarría, M.; Danchin, A.; de Lorenzo, V. From dirt to industrial applications: Pseudomonas putida as a Synthetic Biology chassis for hosting harsh biochemical reactions. Curr. Opin. Chem. Biol. 2016, 34, 20–29. [Google Scholar] [CrossRef]
- Hoover, R.B.; Tang, J. Microbial Extremophiles at the Limits of Life AU - Pikuta, Elena V. Crit. Rev. Microbiol. 2007, 33, 183–209. [Google Scholar] [CrossRef]
- Madigan, M.T.; Orent, A. Thermophilic and halophilic extremophiles. Curr. Opin. Microbiol. 1999, 2, 265–269. [Google Scholar] [CrossRef]
- Schiraldi, C.; De Rosa, M. The production of biocatalysts and biomolecules from extremophiles. Trends Biotechnol. 2002, 20, 515–521. [Google Scholar] [CrossRef]
- Van den Burg, B. Extremophiles as a source for novel enzymes. Curr. Opin. Microbiol. 2003, 6, 213–218. [Google Scholar] [CrossRef]
- Elleuche, S.; Schröder, C.; Sahm, K.; Antranikian, G. Extremozymes—Biocatalysts with unique properties from extremophilic microorganisms. Curr. Opin. Biotechnol. 2014, 29, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mao, J.; Zhang, Z.; Tang, Q.; Xie, Y.; Zhu, J.; Zhang, L.; Liu, Z.; Shi, Y.; Goodfellow, M. Deinococcus wulumuqiensis sp. nov., and Deinococcus xibeiensis sp. nov., isolated from radiation-polluted soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 2006–2010. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Farrance, C.E.; Russell, A.; Yi, H. Reclassification of Deinococcus xibeiensis Wang et al. 2010 as a heterotypic synonym of Deinococcus wulumuqiensis Wang et al. 2010. Int. J. Syst. Evol. Microbiol. 2015, 65, 1083–1085. [Google Scholar] [CrossRef]
- Battista, J.R.; Earl, A.M.; Park, M.-J. Why is Deinococcus radiodurans so resistant to ionizing radiation? Trends Microbiol. 1999, 7, 362–365. [Google Scholar] [CrossRef]
- Xu, X.; Tian, L.; Xu, J.; Xie, C.; Jiang, L.; Huang, H. Analysis and expression of the carotenoid biosynthesis genes from Deinococcus wulumuqiensis R12 in engineered Escherichia coli. AMB Express 2018, 8, 94. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, L.; Zhang, Z.; Shi, Y.; Huang, H. Genome Sequence of a Gamma- and UV-Ray-Resistant Strain, Deinococcus wulumuqiensis R12. Genome Announc. 2013, 1, e00206–e00213. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, Y.; Luo, S.; Suo, Y.; Zhang, S.; Wang, J. Improving cellular robustness and butanol titers of Clostridium acetobutylicum ATCC824 by introducing heat shock proteins from an extremophilic bacterium. J. Biotechnol. 2017, 252, 1–10. [Google Scholar] [CrossRef]
- Wierckx, N.; Koopman, F.; Ruijssenaars, H.J.; de Winde, J.H. Microbial degradation of furanic compounds: Biochemistry, genetics, and impact. Appl. Microbiol. Biotechnol. 2011, 92, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Knaus, T.; Tseliou, V.; Humphreys, L.D.; Scrutton, N.S.; Mutti, F.G. A biocatalytic method for the chemoselective aerobic oxidation of aldehydes to carboxylic acids. Green Chem. 2018, 20, 3931–3943. [Google Scholar] [CrossRef]
- Pang, C.; Zhang, J.; Zhang, Q.; Wu, G.; Wang, Y.; Ma, J. Novel vanillic acid-based poly(ether–ester)s: From synthesis to properties. Polym. Chem. 2015, 6, 797–804. [Google Scholar] [CrossRef]







| Biocatalysts | Reaction Conditions | t (h) | Yield (%) | Ref. |
|---|---|---|---|---|
| Chloroperoxidase | 50 mM HMF, 1 equiv H2O2 per 2 h | 2.5 | 25–40 | [31] |
| Serratia liquefaciens LF14 | 10 mM HMF, 18.2 mg/mL dry cells, in phosphate buffer | 1 | 97 | [30] |
| Immobilized lipase B | 50 mM HMF, 10 mg/mL catalase, addition of aqueous H2O2 (30% v/v) hourly, reaction media: acyl butyrate/tBuOH (1:1 v/v), 40 °C | 24 | 76 | [32] |
| Xanthine oxidase (XO) | 26 mM HMF, 5.6 U E. coli XO, 1.1 mg catalase, phosphate buffer, 37 °C, 150 rpm, air bubbling | 7 | 94 | [29] |
| Comamonas testosterone SC1588 | 160 mM HMF, 30 mg/mL induced microbial cells, phosphate buffer, 20 mM histidine, 150 rpm, 30 °C, tuning pH of the reaction mixture to approximately 7.0 every 24 h. | 36 | 98 | [28] |
| Aldehyde dehydrogenases | 20 mM HMF, 10 µM catalase, 5 µM [NOx], 100 µg/mL [DTT], 0.5 mM [NAD+] , phosphate buffer, 40 °C, 180 rpm | 24 | 91 | [53] |
| D. wulumuqiensis R12 | 300 mM HMF, 200 mg/mL microbial cells, phosphate buffer, 850 rpm, 35 °C, tuning pH of the reaction mixture to approximately 7.0 every 3 h. | 36 | 90 | This work |
| Entry | Substrate | Substrate Concentration (mM) | Product | Yield (%) |
|---|---|---|---|---|
| 1 | Furfural | 100 | Furoic acid | >99 |
| 2 | DFF | 30 | FDCA | >99 |
| 3 | FFCA | 100 | FDCA | 63 |
| 4 | Vanillin | 5 | Vanillic acid | >99 |
| 5 | p-Hydroxybenzaldehyde | 5 | 4-Hydroxybenzoic acid | 70 |
| 6 | Terephthaldehyde | 2 | Terephthalic acid | >99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cang, R.; Shen, L.-Q.; Yang, G.; Zhang, Z.-D.; Huang, H.; Zhang, Z.-G. Highly Selective Oxidation of 5-Hydroxymethylfurfural to 5-Hydroxymethyl-2-Furancarboxylic Acid by a Robust Whole-Cell Biocatalyst. Catalysts 2019, 9, 526. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9060526
Cang R, Shen L-Q, Yang G, Zhang Z-D, Huang H, Zhang Z-G. Highly Selective Oxidation of 5-Hydroxymethylfurfural to 5-Hydroxymethyl-2-Furancarboxylic Acid by a Robust Whole-Cell Biocatalyst. Catalysts. 2019; 9(6):526. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9060526
Chicago/Turabian StyleCang, Ran, Li-Qun Shen, Guang Yang, Zhi-Dong Zhang, He Huang, and Zhi-Gang Zhang. 2019. "Highly Selective Oxidation of 5-Hydroxymethylfurfural to 5-Hydroxymethyl-2-Furancarboxylic Acid by a Robust Whole-Cell Biocatalyst" Catalysts 9, no. 6: 526. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9060526