Two-Step Production of Neofructo-Oligosaccharides Using Immobilized Heterologous Aspergillus terreus 1F-Fructosyltransferase Expressed in Kluyveromyces lactis and Native Xanthophyllomyces dendrorhous G6-Fructosyltransferase
Abstract
:1. Introduction
2. Results and Discussion
2.1. FOS Production by Native and Recombinant Fructosyltransferases
2.2. Enzyme Characterization
2.2.1. Characterization of 1-FFT
2.2.2. Characterization of G6-FFT
2.2.3. Analysis of 6-SFT by-Product Formation
2.3. Immobilization of 1-FFT
2.3.1. Screening Immobilization Carriers and Conditions
2.3.2. Optimization of 1-FFT Immobilization
2.3.3. FOS Production with Free and Immobilized 1-FFT
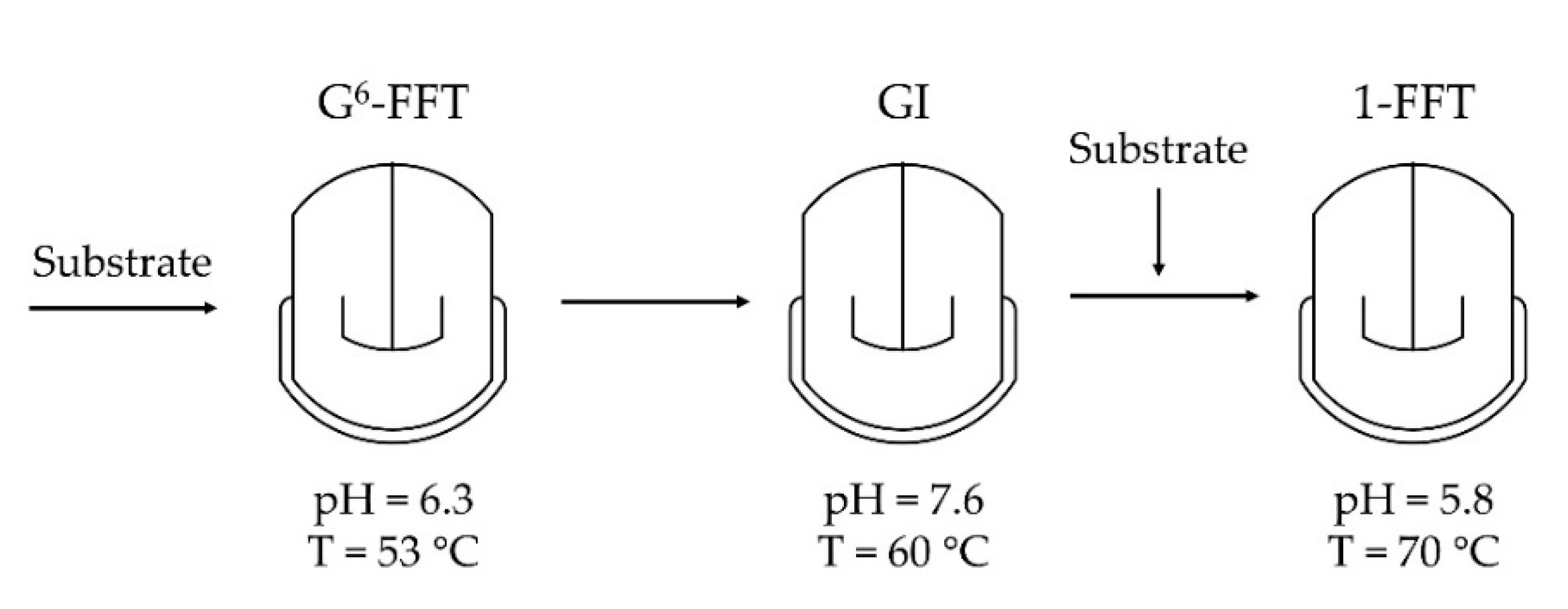
2.4. Two-Step Microscale neoFOS Production
2.5. Two-Step 100-mL Scale neoFOS Production
3. Materials and Methods
3.1. Microbial Strains, Reagents and Materials
3.2. Enzyme Production
3.3. Concentration of 6-SFT and G6-FFT Solutions
3.4. Enzyme Reactions
3.5. Enzyme Immobilization
3.6. Modification of Epoxy Carrier
3.7. Two-Step Microscale neoFOS Production
3.8. Two-Step 100-mL Scale neoFOS Production
3.9. UHPLC Analytics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiang, X.; YongLie, C.; QianBing, W. Health benefit application of functional oligosaccharides. Carbohydr. Polym. 2009, 77, 435–441. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Rastall, R.A. Prebiotics in foods. Curr. Opin. Biotechnol. 2012, 23, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.P.; Gonçalves, M.D.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [Green Version]
- Roberfroid, M.B. Inulin-type fructans: Functional food ingredients. J. Nutr. 2007, 137, 2493S–2502S. [Google Scholar] [CrossRef] [PubMed]
- Erdös, B.; Grachten, M.; Czermak, P.; Kovács, Z. Artificial Neural Network-Assisted Spectrophotometric Method for Monitoring Fructo-oligosaccharides Production. Food Bioprocess Technol. 2017, 11, 305–313. [Google Scholar] [CrossRef]
- Spohner, S.C.; Schaum, V.; Quitmann, H.; Czermak, P. Kluyveromyces lactis: An emerging tool in biotechnology. J. Biotechnol. 2016, 222, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Benjamins, E.; Grau, K.; Ur Rehman, A.; Ebrahimi, M.; Czermak, P. Recent developments in manufacturing oligosaccharides with prebiotic functions. Adv. Biochem. Eng. Biotechnol. 2014, 143, 257–295. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Kovacs, Z.; Quitmann, H.; Ebrahimi, M.; Czermak, P. Enzymatic production of fructo-oligosaccharides from inexpensive and abundant substrates using a membrane reactor system. Sep. Sci. Technol. 2016, 58, 548. [Google Scholar] [CrossRef]
- Hidaka, H.; Eida, T.; Takizawa, T.; Tokunaga, T.; Tashiro, Y. Effects of Fructooligosaccharides on Intestinal Flora and Human Health. Bifidobact. Microflora 1986, 5, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Kilian, S.; Kritzinger, S.; Rycroft, C.; Gibson, G.; Du Preez, J. The effects of the novel bifidogenic trisaccharide, neokestose, on the human colonic microbiota. World J. Microbiol. Biotechnol. 2002, 18, 637–644. [Google Scholar] [CrossRef]
- Spohner, S.C.; Czermak, P. Enzymatic production of prebiotic fructo-oligosteviol glycosides. J. Mol. Catal. B Enzym. 2016, 131, 79–84. [Google Scholar] [CrossRef]
- Spohner, S.C.; Czermak, P. Heterologous expression of Aspergillus terreus fructosyltransferase in Kluyveromyces lactis. New Biotechnol. 2016, 33, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W. Fructooligosaccharides—Occurrence, preparation, and application. Enzym. Microb. Technol. 1996, 19, 107–117. [Google Scholar] [CrossRef]
- Hidaka, H.; Hirayama, M.; Yamada, K. Review Article: Fructooligosaccharides Enzymatic Preparation and Biofunctions. J. Carbohydr. Chem. 1991, 10, 509–522. [Google Scholar] [CrossRef]
- Simmering, R.; Blaut, M. Pro- and prebiotics—The tasty guardian angels? Appl. Microbiol. Biotechnol. 2001, 55, 19–28. [Google Scholar] [CrossRef]
- Lim, J.S.; Lee, J.H.; Kang, S.W.; Park, S.W.; Kim, S.W. Studies on production and physical properties of neo-FOS produced by co-immobilized Penicillium citrinum and neo-fructosyltransferase. Eur. Food Res. Technol. 2007, 225, 457–462. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5, 73–98. [Google Scholar] [CrossRef]
- Gibson, G.; Wang, X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J. Appl. Bacteriol. 1994, 77, 412–420. [Google Scholar] [CrossRef]
- Linde, D.; Rodríguez-Colinas, B.; Estévez, M.; Poveda, A.; Plou, F.J.; Lobato, M.F. Analysis of neofructooligosaccharides production mediated by the extracellular β-fructofuranosidase from Xanthophyllomyces dendrorhous. Bioresour. Technol. 2012, 109, 123–130. [Google Scholar] [CrossRef]
- Cruz, R.; Cruz, V.D.; Belini, M.Z.; Belote, J.G.; Vieira, C.R. Production of fructooligosaccharides by the mycelia of Aspergillus japonicus immobilized in calcium alginate. Bioresour. Technol. 1998, 65, 139–143. [Google Scholar] [CrossRef]
- Chuankhayan, P.; Hsieh, C.-Y.; Huang, Y.-C.; Hsieh, Y.-Y.; Guan, H.-H.; Hsieh, Y.-C.; Tien, Y.-C.; Chen, C.-D.; Chiang, C.-M.; Chen, C.-J. Crystal Structures of Aspergillus japonicus Fructosyltransferase Complex with Donor/Acceptor Substrates Reveal Complete Subsites in the Active Site for Catalysis. J. Boil. Chem. 2010, 285, 23251–23264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashby, R.; Hjortkjaer, R.; Stavnsbjerg, M.; Gürtler, H.; Pedersen, P.; Bootman, J.; Hudson-Walker, G.; Tesh, J.; Willoughby, C.; West, H.; et al. Safety evaluation of Streptomyces murinus glucose isomerase. Toxicol. Lett. 1987, 36, 23–35. [Google Scholar] [CrossRef]
- Skoet, G.; Guertler, H. Xylose Isomerase (Glucose Isomerase) from Streptomyces Murinus Cluster. U.S. Patent US4687742A, 18 August 1987. [Google Scholar]
- Jørgensen, O.B.; Karlsen, L.G.; Nielsen, N.B.; Pedersen, S.; Rugh, S. A New Immobilized Glucose Isomerase with High Productivity Produced by a Strain ofStreptomyces murinus. Starch Stärke 1988, 40, 307–313. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C. Enzymatic and whole cell catalysis: Finding new strategies for old processes. Biotechnol. Adv. 2011, 29, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Lorenzoni, A.S.; Aydos, L.F.; Klein, M.P.; Rodrigues, R.C.; Hertz, P.F. Fructooligosaccharides synthesis by highly stable immobilized β-fructofuranosidase from Aspergillus aculeatus. Carbohydr. Polym. 2014, 103, 193–197. [Google Scholar] [CrossRef]
- Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J.M. Guidelines and Cost Analysis for Catalyst Production in Biocatalytic Processes. Org. Process. Res. Dev. 2011, 15, 266–274. [Google Scholar] [CrossRef]
- Nelson, J.M.; Griffin, E.G. Adsorption of Invertase. J. Am. Chem. Soc. 1916, 38, 1109–1115. [Google Scholar] [CrossRef]
- Mateo, C.; Grazu, V.; Pessela, B.; Montes, T.; Palomo, J.; Torres, R.T.R.; Gallego, F.L.; Fernandez-Lafuente, R.; Guisan, J. Advances in the design of new epoxy supports for enzyme immobilization-stabilization. Biochem. Soc. Trans. 2007, 35, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Grazu, V.; Palomo, J.M.; López-Gallego, F.; Fernandez-Lafuente, R.; Guisan, J.M. Immobilization of enzymes on heterofunctional epoxy supports. Nat. Protoc. 2007, 2, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Engel, L.; Ebrahimi, M.; Czermak, P. Membrane chromatography reactor system for the continuous synthesis of galactosyl-oligosaccharides. Desalination 2008, 224, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Míguez, N.; Gimeno-Pérez, M.; Fernández-Polo, D.; Cervantes, F.V.; Ballesteros, A.O.; Fernández-Lobato, M.; Ribeiro, M.H.; Plou, F.J. Immobilization of the β-fructofuranosidase from Xanthophyllomyces dendrorhous by Entrapment in Polyvinyl Alcohol and Its Application to Neo-Fructooligosaccharides Production. Catalysts 2018, 8, 201. [Google Scholar] [CrossRef]
- Linde, D.; Macias, I.; Fernández-Arrojo, L.; Plou, F.J.; Jiménez, A.; Fernández-Lobato, M. Molecular and biochemical characterization of a beta-fructofuranosidase from Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2009, 75, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Wang, J.; Chen, J.; Yang, N.; Jin, Z.; Xu, X. Production of neo-fructooligosaccharides using free-whole-cell biotransformation by Xanthophyllomyces dendrorhous. Bioresour. Technol. 2010, 101, 7472–7478. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, I.; De Segura, A.G.; Fernández-Arrojo, L.; Alcalde, M.; Yates, M.; Rojas-Cervantes, M.L.; Plou, F.J.; Ballesteros, A. Immobilisation of fructosyltransferase from Aspergillus aculeatus on epoxy-activated Sepabeads EC for the synthesis of fructo-oligosaccharides. J. Mol. Catal. B Enzym. 2005, 35, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, M.; Sumi, N.; Hidaka, H. Purification and Properties of a Fructooligosaccharide-producing β-Fructofuranosidase from Aspergillus niger ATCC 20611. Agric. Boil. Chem. 1989, 53, 667–673. [Google Scholar] [CrossRef]
- Kushi, R.T.; Monti, R.; Contiero, J. Production, purification and characterization of an extracellular inulinase from Kluyveromyces marxianus var. bulgaricus. J. Ind. Microbiol. Biotechnol. 2000, 25, 63–69. [Google Scholar] [CrossRef]
- Guo, W.; Yang, H.; Qiang, S.; Fan, Y.; Shen, W.; Chen, X. Overproduction, purification, and property analysis of an extracellular recombinant fructosyltransferase. Eur. Food Res. Technol. 2016, 242, 1159–1168. [Google Scholar] [CrossRef]
- Cruz-Guerrero, A.E.; Olvera, J.L.; García-Garibay, M.; Gómez-Ruiz, L. Inulinase-hyperproducing strains of Kluyveromyces sp. isolated from aguamiel (Agave sap) and pulque. World J. Microbiol. Biotechnol. 2006, 22, 115–117. [Google Scholar] [CrossRef]
- Dworschack, R.G.; Wickerham, L.J. Production of Extracellular and Total Invertase by Candida utilis, Saccharomyces cerevisiae, and Other Yeasts. Appl. Microbiol. 1961, 9, 291–294. [Google Scholar] [PubMed]
- Rouwenhorst, R.J.; Visser, L.E.; Van Der Baan, A.A.; Scheffers, W.A.; Van Dijken, J.P. Production, Distribution, and Kinetic Properties of Inulinase in Continuous Cultures of Kluyveromyces marxianus CBS 6556. Appl. Environ. Microbiol. 1988, 54, 1131–1137. [Google Scholar] [PubMed]
- Rouwenhorst, R.J.; Ritmeester, W.S.; Scheffers, W.A.; Van Dijken, J.P. Localization of inulinase and invertase in Kluyveromyces species. Appl. Environ. Microbiol. 1990, 56, 3329–3336. [Google Scholar] [PubMed]
- Álvaro-Benito, M.; De Abreu, M.; Fernández-Arrojo, L.; Plou, F.J.; Jiménez-Barbero, J.; Ballesteros, A.; Polaina, J.; Fernández-Lobato, M. Characterization of a β-fructofuranosidase from Schwanniomyces occidentalis with transfructosylating activity yielding the prebiotic 6-kestose. J. Biotechnol. 2007, 132, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Farine, S.; Versluis, C.; Bonnici, P.; Heck, A.; L’Hommé, C.; Puigserver, A.; Biagini, A. Application of high performance anion exchange chromatography to study invertase-catalysed hydrolysis of sucrose and formation of intermediate fructan products. Appl. Microbiol. Biotechnol. 2001, 55, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, L.; More, S.S. Bacterial invertases: Occurrence, production, biochemical characterization, and significance of transfructosylation. J. Basic Microbiol. 2017, 57, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Babich, L.; Hartog, A.F.; Van Der Horst, M.A.; Wever, R. Continuous-Flow Reactor-Based Enzymatic Synthesis of Phosphorylated Compounds on a Large Scale. Chem. Eur. J. 2012, 18, 6604–6609. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, D.; Wang, W.; Durrani, R.; Yang, B.; Wang, Y. Immobilization of SMG1-F278N lipase onto a novel epoxy resin: Characterization and its application in synthesis of partial glycerides. J. Mol. Catal. B Enzym. 2016, 133, 154–160. [Google Scholar] [CrossRef]
- Weaver, J.; Husson, S.M.; Murphy, L.; Wickramasinghe, S.R. Anion exchange membrane adsorbers for flow-through polishing steps: Part II. Virus, host cell protein, DNA clearance, and antibody recovery. Biotechnol. Bioeng. 2013, 110, 500–510. [Google Scholar] [CrossRef]
- Cruz, I.B.; Jorge, R.F.; Castro, P.M.L.; Ferraro, V.; Pintado, M.M. Effects of Physical Parameters onto Adsorption of the Borderline Amino Acids Glycine, Lysine, Taurine, and Tryptophan upon Amberlite XAD16 Resin. J. Chem. Eng. Data 2013, 58, 707–717. [Google Scholar]
- Ghanem, F. Juice Debittering: Basic Science, Optimization, and Recent Advances. In Proceedings of the ASME 2012 Citrus Engineering Conference, Lake Alfred, FL, USA, 15 March 2012; p. 1, ISBN 978-0-7918-9998-4. [Google Scholar]
- Zhang, Y.-W.; Tiwari, M.K.; Jeya, M.; Lee, J.-K. Covalent immobilization of recombinant Rhizobium etli CFN42 xylitol dehydrogenase onto modified silica nanoparticles. Appl. Microbiol. Biotechnol. 2011, 90, 499–507. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Sheng, J.; Sun, M. Immobilization of Yarrowia lipolytica Lipase on Macroporous Resin Using Different Methods: Characterization of the Biocatalysts in Hydrolysis Reaction. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar]
- Gross, D.; Blanchard, P.H.; Bell, D.J. neoKestose: A trisaccharide formed from sucrose by yeast invertase. J. Chem. Soc. 1954, 1727–1730. [Google Scholar] [CrossRef]
- Gutiérrez-Alonso, P.; Fernández-Arrojo, L.; Plou, F.J.; Fernández-Lobato, M. Biochemical characterization of a β-fructofuranosidase fromRhodotorula dairenensiswith transfructosylating activity. FEMS Yeast Res. 2009, 9, 768–773. [Google Scholar] [CrossRef]
- Grizard, D.; Barthomeuf, C. Enzymatic synthesis and structure determination of NEO-FOS. Food Biotechnol. 1999, 13, 93–105. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Xu, X.; Ning, Y.; Jin, Z.; Tian, Y. Biochemical characterization of an intracellular 6G-fructofuranosidase from Xanthophyllomyces dendrorhous and its use in production of neo-fructooligosaccharides (neo-FOSs). Bioresour. Technol. 2011, 102, 1715–1721. [Google Scholar] [CrossRef]
- Sheu, D.-C.; Chang, J.-Y.; Chen, Y.-J.; Lee, C.-W. Production of high-purity neofructooligosaccharides by culture of Xanthophyllomyces dendrorhous. Bioresour. Technol. 2013, 132, 432–435. [Google Scholar] [CrossRef] [Green Version]
- Burghardt, J.P.; Oestreich, A.M.; Weidner, T.; Gerlach, D.; Czermak, P. Development of a Chemically Defined Fermentation Medium for the Production of a New Recombinant Fructosyltransferase. Int. J. Pharma Med. Biol. Sci. 2018, 7. [Google Scholar] [CrossRef]
- Gimeno-Pérez, M.; Linde, D.; Fernández-Arrojo, L.; Plou, F.J.; Fernández-Lobato, M. Heterologous overproduction of β-fructofuranosidase from yeast Xanthophyllomyces dendrorhous, an enzyme producing prebiotic sugars. Appl. Microbiol. Biotechnol. 2015, 99, 3459–3467. [Google Scholar] [CrossRef]





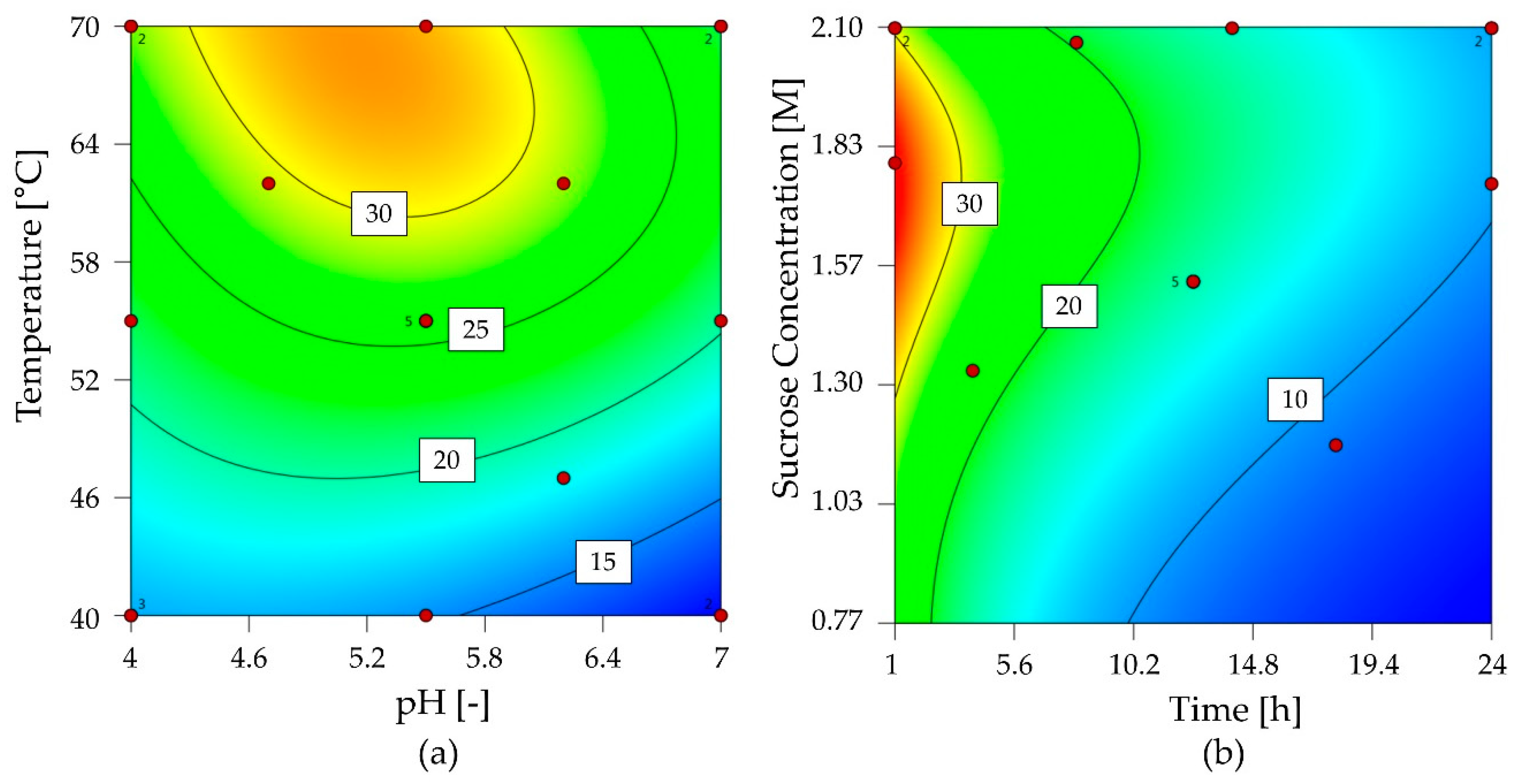

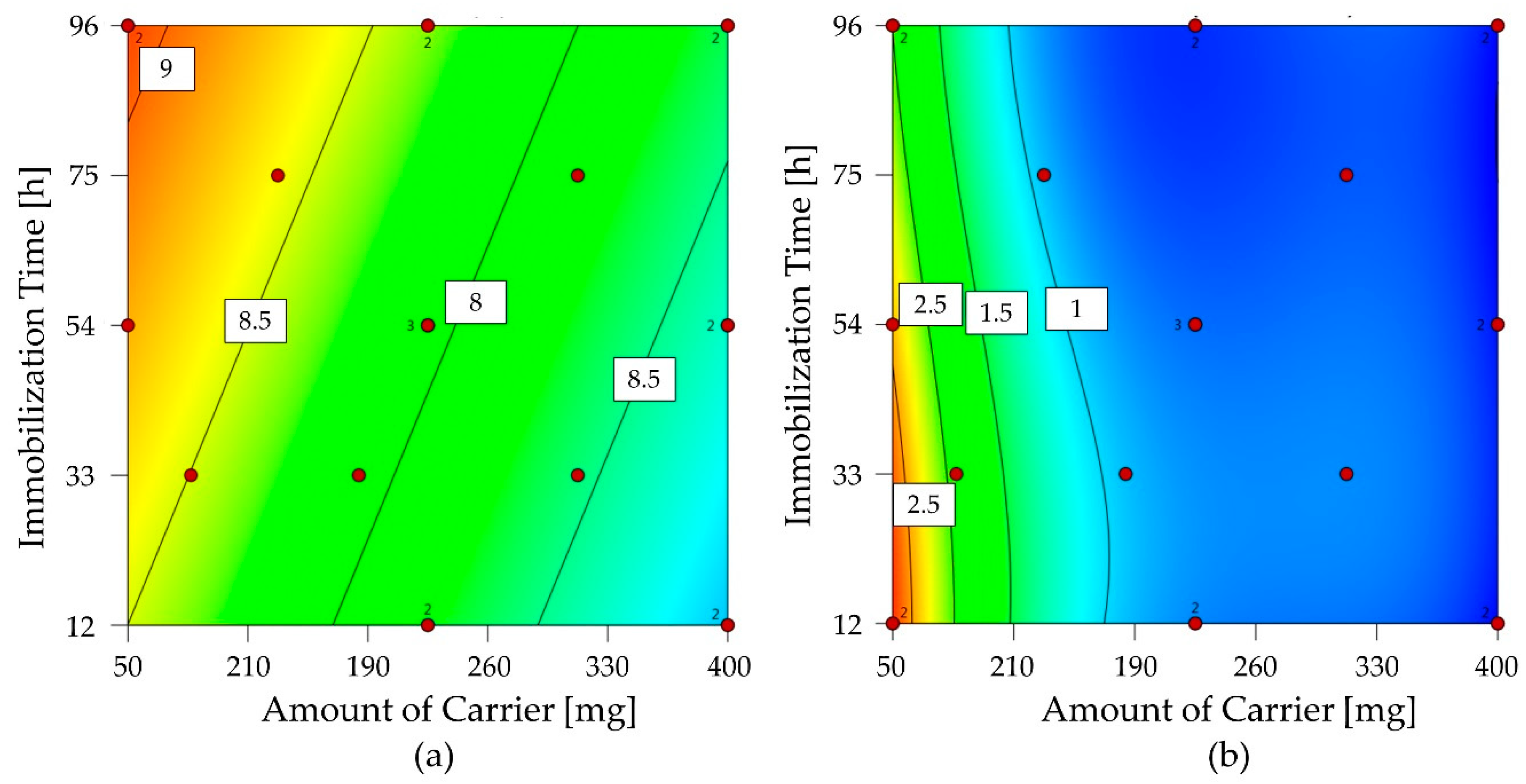
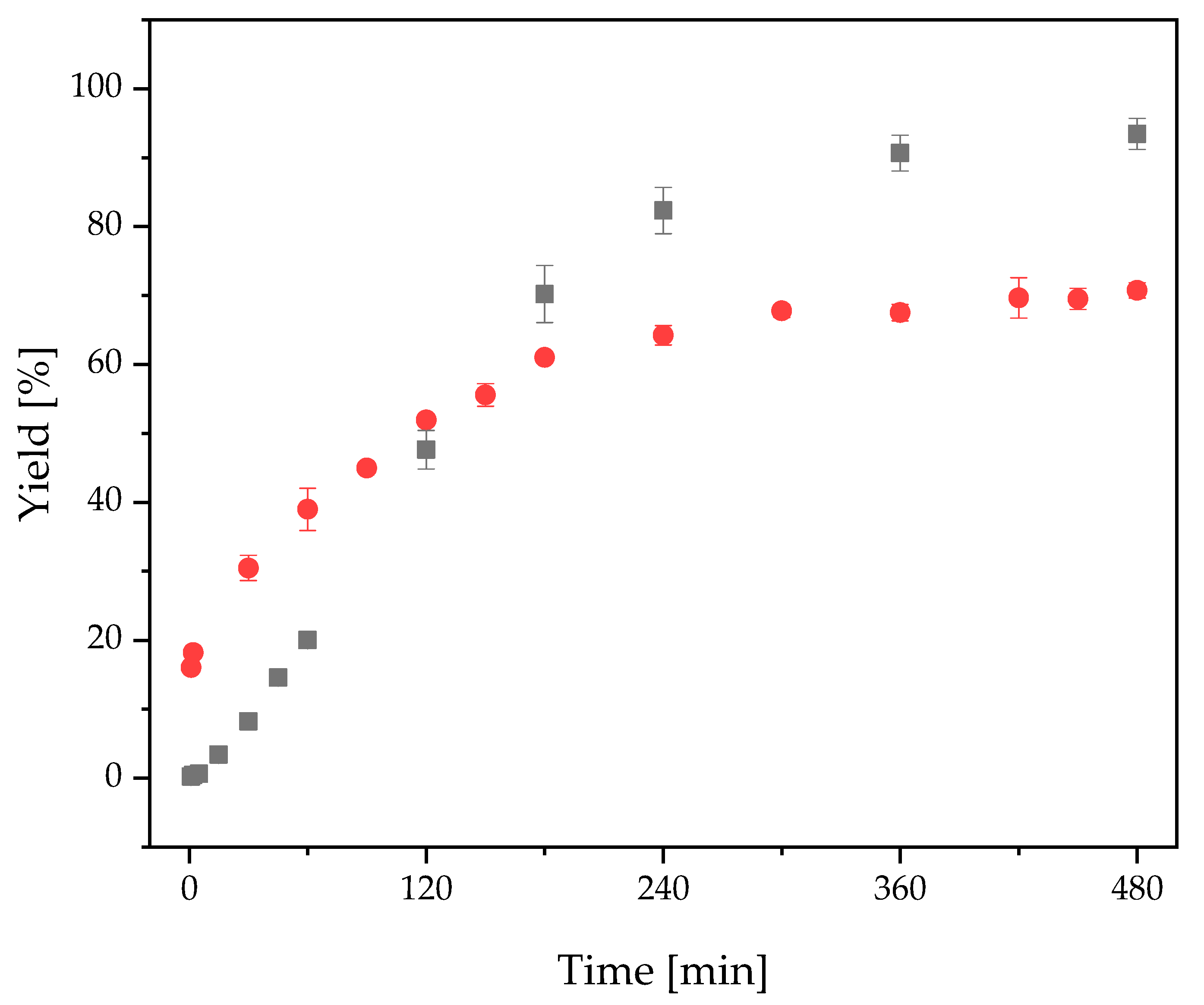

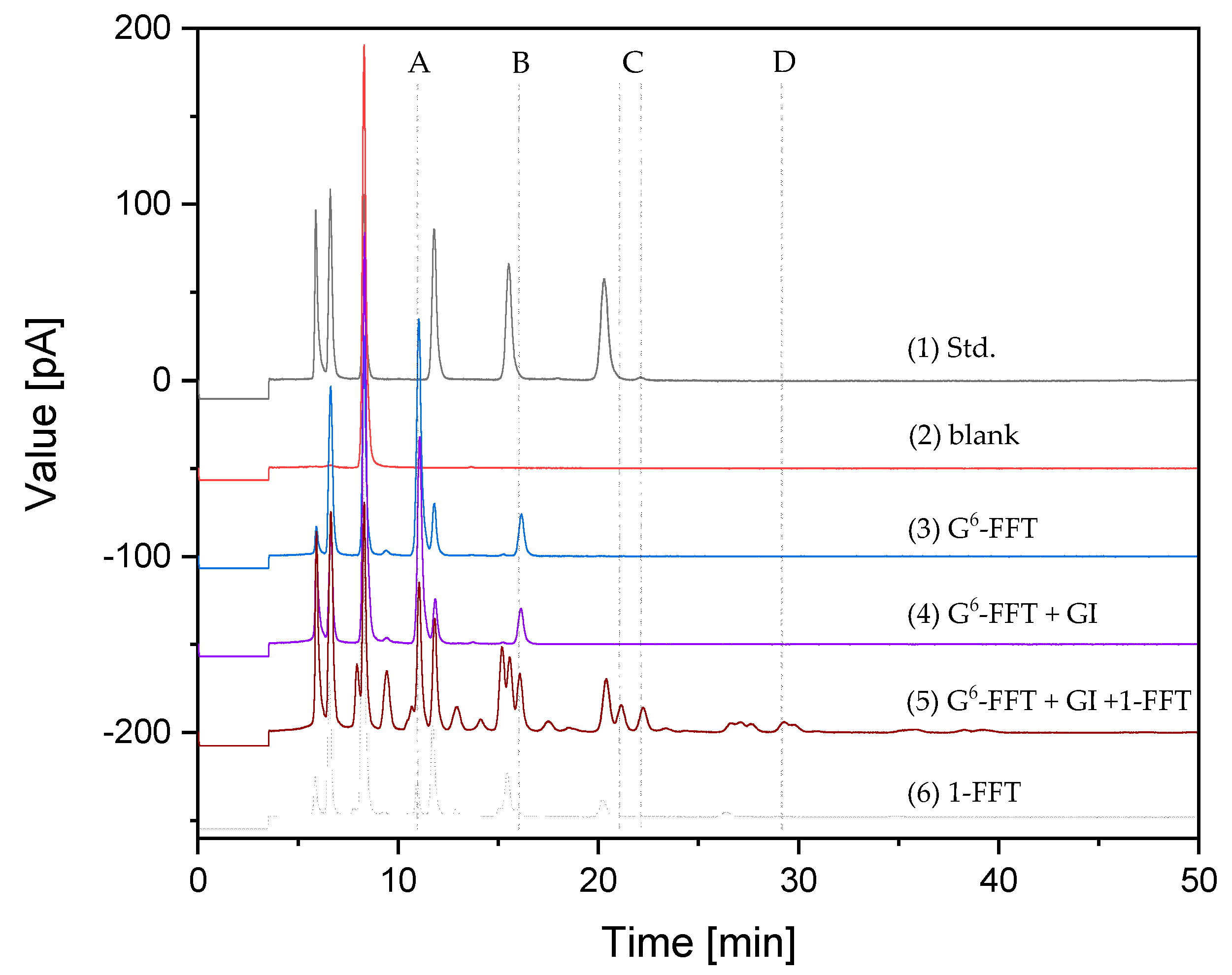
| Process Step | Sucrose GF [mM] | Glucose G [mM] | Neonystose GF3 [mM] | Neoseries GF4 [mM] | YFOS,total [%] |
|---|---|---|---|---|---|
| (A) Blank | 1849.80 ± 113.89 | <LOD | <LOD | <LOD | - |
| (B) G6-FFT | 501.56 ± 42.41 | 755.33 ± 51.51 | 33.05 ± 3.01 | <LOD | 68.55 ± 4.11 |
| (C) GI | 529.94 ± 23.64 | 436.17 ± 37.61 | 32.51 ± 0.49 | <LOD | 68.69 ± 2.93 |
| (D) Sucrose | 1916.78 ± 136.67 | 435.28 ± 41.46 | 30.04 ± 4.47 | <LOD | 31.08 ± 1.88 † |
| (E) 1-FFT | 600.72 ± 86.88 | 1235.15 ± 59.60 | 53.07 ± 1.66 | 20.8 ± 1.91 | 47.02 ± 3.02 † |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burghardt, J.P.; Baas, M.; Gerlach, D.; Czermak, P. Two-Step Production of Neofructo-Oligosaccharides Using Immobilized Heterologous Aspergillus terreus 1F-Fructosyltransferase Expressed in Kluyveromyces lactis and Native Xanthophyllomyces dendrorhous G6-Fructosyltransferase. Catalysts 2019, 9, 673. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9080673
Burghardt JP, Baas M, Gerlach D, Czermak P. Two-Step Production of Neofructo-Oligosaccharides Using Immobilized Heterologous Aspergillus terreus 1F-Fructosyltransferase Expressed in Kluyveromyces lactis and Native Xanthophyllomyces dendrorhous G6-Fructosyltransferase. Catalysts. 2019; 9(8):673. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9080673
Chicago/Turabian StyleBurghardt, Jan Philipp, Markus Baas, Doreen Gerlach, and Peter Czermak. 2019. "Two-Step Production of Neofructo-Oligosaccharides Using Immobilized Heterologous Aspergillus terreus 1F-Fructosyltransferase Expressed in Kluyveromyces lactis and Native Xanthophyllomyces dendrorhous G6-Fructosyltransferase" Catalysts 9, no. 8: 673. https://0-doi-org.brum.beds.ac.uk/10.3390/catal9080673






