One-Pot Synthesis, X-ray Single Crystal and Molecular Insight of Enaminone-Based β-Morpholino-/N-Methylpiperazinyl-/Pyrrolidinylpropiophenone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Enaminones 1–3



2.2. Computational Methods
3. Results and Discussion
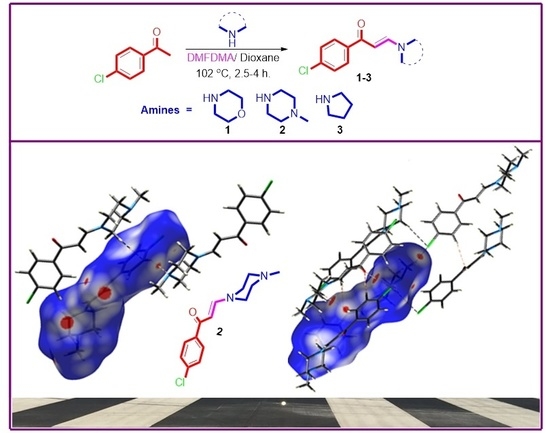
3.1. Synthesis of Enaminones 1–3
3.2. X-ray Structure of 1, and 2
3.3. Analysis of Molecular Packing
3.4. DFT Studies
Geometric Parameters
3.5. Reactivity Studies
3.6. NMR and UV-Vis Electronic Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riyadh, S.M. Enaminones as building blocks for the synthesis of substituted pyrazoles with antitumor and antimicrobial activities. Molecules 2011, 16, 1834–1853. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, S.K.; Shaban, S.S.; Hashem, A.I. Facile and expedient synthesis and anti-proliferative activity of diversely pyrrolones bearing 1, 3-diphenylpyrazole moiety. Synth. Commun. 2020, 50, 185–196. [Google Scholar] [CrossRef]
- Dannhardt, G.; Bauer, A.; Nowe, U. Non-Steroidal Anti-Inflammatory Agents, Part 24 [1] Pyrrolidino Enaminones as Models to Mimic Arachidonic Acid. Archiv der Pharmazie 1997, 330, 74–82. [Google Scholar] [CrossRef]
- Khurana, M.; Salama, N.N.; Scott, K.R.; Nemieboka, N.N.; Bauer, K.S.; Eddington, N.D. Preclinical evaluation of the pharmacokinetics, brain uptake and metabolism of E121, an antiepileptic enaminone ester, in rats. Biopharm. Drug Dispos. 2003, 24, 397–407. [Google Scholar] [CrossRef]
- Edafiogho, I.O.; Ananthalakshmi, K.V.V.; Kombian, S.B. Anticonvulsant evaluation and mechanism of action of benzylamino enaminones. Bioorg. Med. Chem. 2006, 14, 5266–5272. [Google Scholar] [CrossRef]
- Michael, J.P.; De Koning, C.B.; Hosken, G.D.; Stanbury, T.V. Reformatsky reactions with N-arylpyrrolidine-2-thiones: Synthesis of tricyclic analogues of quinolone antibacterial agents. Tetrahedron 2001, 57, 9635–9648. [Google Scholar] [CrossRef]
- El-Borai, M.A.; Rizk, H.F.; Beltagy, D.M.; El-Deeb, I.Y. Microwave-Assisted synthesis of some new pyrazolopyridines and their antioxidant, antitumor and antimicrobial activities. Eur. J. Med. Chem. 2013, 66, 415–422. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Abo-Ashour, M.F.; Berrino, E.; Vullo, D.; Ghabbour, H.A.; Al-Rashood, S.T.; Hassan, G.S.; Alkahtani, H.M.; Almehizia, A.A.; Alharbi, A.; et al. SLC-0111 enaminone analogs, 3/4-(3-aryl-3-oxopropenyl) aminobenzenesulfonamides, as novel selective subnanomolar inhibitors of the tumor-associated carbonic anhydrase isoform IX. Bioorg. Chem. 2019, 83, 549–558. [Google Scholar] [CrossRef]
- Ali, M.; Barakat, A.; El-Faham, A.; Al-Rasheed, H.H.; Dahlous, K.; Al-Majid, A.M.; Sharma, A.; Yousuf, S.; Sanam, M.; Ul-Haq, Z.; et al. Synthesis and characterisation of thiobarbituric acid enamine derivatives, and evaluation of their α-glucosidase inhibitory and anti-glycation activity. Enzyme Inhib. Med. Chem. 2020, 35, 692–701. [Google Scholar] [CrossRef]
- Chaaban, I.; Greenhill, J.V.; Akhtar, P. Enaminones in the mannich reaction. Part 2. Further investigations of internal mannich reactions. J. Chem. Soc. Perkin Trans. 1 1979, 6, 1593–1596. [Google Scholar] [CrossRef]
- Michael, J.P.; De Koning, C.B.; Gravestock, D.; Hosken, G.D.; Howard, A.S.; Jungmann, C.M.; Krause, R.; Parsons, A.S.; Pelly, S.; Stanbury, T. Enaminones: Versatile intermediates for natural product synthesis. Purr. Appl. Chem. 1999, 71, 979–988. [Google Scholar] [CrossRef]
- Spivey, A.C.; Srikaran, R.; Diaper, C.M.; Turner, D.J. Traceless solid phase synthesis of 2-substituted pyrimidines using an “off-The-Shelf” chlorogermane-functionalised resin. Org. Biomol. Chem. 2003, 1, 1638–1640. [Google Scholar] [CrossRef]
- Souza, F.R.; Souza, V.T.; Ratzlaff, V.; Borges, L.P.; Oliveira, M.R.; Bonacorso, H.G.; Zanatta, N.; Martins, M.A.P.; Mello, C.F. Hypothermic and antipyretic effects of 3-methyl- and 3-phenyl-5-hydroxy-5-trichloromethyl-4,5-dihydro-1H-pyrazole-1-carboxyamides in mice. Eur. J. Pharmacol. 2002, 451, 141–147. [Google Scholar] [CrossRef]
- Wang, Y.F.; Izawa, T.; Kobayashi, S.; Ohno, M. Stereocontrolled synthesis of (+)-negamycin from an acyclic homoallylamine by 1,3-asymmetric induction. J. Amer. Chem. Soc. 1982, 104, 6465–6466. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yin, L.; Wang, Y. A General and Efficient Method for the Preparation of β-Enamino Ketones and Esters Catalyzed by Indium Tribromide. Adv. Synth. Catal. 2006, 348, 184–190. [Google Scholar] [CrossRef]
- Laskar, R.A.; Begum, N.A.; Mir, M.H.; Ali, S.; Khan, A.T. Vanadium (IV) acetylacetonate catalyzed stereoselective synthesis of β-enaminoesters and β-enaminones. Tetrahedron Lett. 2013, 54, 436–440. [Google Scholar] [CrossRef]
- Lenin, R.; Raju, R.M. Lanthanum trichloride: An efficient Lewis acid catalyst for chemo and regioselective enamination of β-dicarbonyl compounds. Arkivoc 2007, 13, 204–209. [Google Scholar]
- Yu, X.; Wang, L.; Feng, X.; Bao, M.; Yamamoto, Y. Copper-Catalyzed aldol-Type addition of ketones to aromatic nitriles: A simple approach to enaminone synthesis. Chem. Commun. 2013, 49, 2885–2887. [Google Scholar] [CrossRef]
- Eshghi, H.; Seyedi, S.M.; Safaei, E.; Vakili, M.; Farhadipour, A.; Bayat-Mokhtari, M. Silica supported Fe(HSO4)3 as an efficient, heterogeneous and recyclable catalyst for synthesis of β-enaminones and β-enamino esters. J. Mol. Catal. A Chem. 2012, 363, 430–436. [Google Scholar] [CrossRef]
- Bhatte, K.D.; Tambade, P.J.; Dhake, K.P.; Bhanage, B.M. Silver nanoparticles as an efficient, heterogeneous and recyclable catalyst for synthesis of β-enaminones. Cat. Commun. 2010, 11, 1233–1237. [Google Scholar] [CrossRef]
- Rout, L.; Kumar, A.; Dhaka, R.S.; Dash, P. Bimetallic Ag–Cu alloy nanoparticles as a highly active catalyst for the enamination of 1, 3-dicarbonyl compounds. RSC Adv. 2016, 6, 49923–49940. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17 (2017) University of Western Australia. Available online: https://crystalexplorer.scb.uwa.edu.au/ (accessed on 7 April 2020).
- Chen, J.; Zhang, Z.; Liu, S.; Yang, C.; Xia, C. One-Pot tandem synthesis of 2, 3-Unsubstituted indoles, an improved Leimgruber–Batchoindole synthesis. RSC Adv. 2014, 4, 4672–4675. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Revision A02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Marten, B.; Kim, K.; Cortis, C.; Friesner, R.A.; Murphy, R.B.; Ringnalda, M.N.; Sitkoff, D.; Honig, B. New Model for Calculation of Solvation Free Energies: Correction of Self-Consistent Reaction Field Continuum Dielectric Theory for Short-Range Hydrogen-Bonding Effects. J. Phys. Chem. 1996, 100, 11765–11775. [Google Scholar] [CrossRef]
- Tannor, D.J.; Marten, B.; Murphy, R.; Friesner, R.A.; Sitkoff, D.; Nicholls, A.; Ringnalda, M.; Goddard, W.A.; Honig, B. Accurate first principles calculation of molecular charge distributions and solvation energies from ab initio quantum mechanics and continuum dielectric theory. J. Am. Chem. Soc. 1994, 116, 11875–11882. [Google Scholar] [CrossRef]
- Li, M.; Fang, D.; Geng, F.; Dai, X. Silver-Catalyzed efficient synthesis of enaminones from propargyl alcohols and amines. Tetrahedron Lett. 2017, 58, 4747–4749. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Chang, R. Chemistry, 7th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kosar, B.; Albayrak, C. Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino) methyl] phenol. Spectrochim. Acta 2011, 78, 160–167. [Google Scholar] [CrossRef]
- Koopmans, T.A. Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Singh, R.N.; Kumar, A.; Tiwari, R.K.; Rawat, P.; Gupta, V.P. A combined experimental and quantum chemical (DFT and AIM) study on molecular structure, spectroscopic properties, NBO and multiple interaction analysis in a novel ethyl 4-[2-(carbamoyl) hydrazinylidene]-3,5-dimethyl-1H-pyrrole-2-carboxylate and its dimer. J. Mol. Strut. 2013, 1035, 427–440. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A Comparison of Models for Calculating Nuclear Magnetic Resonance Shielding Tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]


















| Bond | Distance |
|---|---|
| Cl(1)-C(3) | 1.7466(15) |
| O(1)-C(7) | 1.2403(19) |
| O(2)-C(12) | 1.421(2) |
| O(2)-C(11) | 1.422(2) |
| N(1)-C(9) | 1.338(2) |
| N(1)-C(10) | 1.460(2) |
| N(1)-C(13) | 1.462(2) |
| Bonds | Angles |
|---|---|
| Cl(1)-C(3) | 1.7427(17) |
| O(1)-C(7) | 1.2419(19) |
| N(1)-C(9) | 1.328(2) |
| N(1)-C(10) | 1.460(2) |
| N(1)-C(14) | 1.462(2) |
| N(2)-C(13) | 1.450(2) |
| N(2)-C(11) | 1.458(2) |
| N(2)-C(12) | 1.460(2) |
| Parameter | 1 | 2 |
|---|---|---|
| HOMO | −5.7664 | −5.6412 |
| LUMO | −1.4368 | −1.3339 |
| I | 5.7664 | 5.6412 |
| A | 1.4368 | 1.3339 |
| η | 4.3296 | 4.3073 |
| μ | −3.6016 | −3.4876 |
| ω | 1.4980 | 1.4119 |
| 1 | 2 | ||||
|---|---|---|---|---|---|
| Λ | f | Major Contributions | λ | F | Major Contributions |
| 326.5 | 0.520 | HOMO→LUMO (82%), H-1→LUMO (16%) | 327.0 | 0.500 | HOMO→LUMO (79%), H-2→LUMO (19%) |
| 254.8 | 0.402 | H-2→LUMO (83%) | 270.3 | 0.014 | HOMO→L + 1 (94%) |
| 209.9 | 0.116 | H-3→LUMO (18%), H-3→L + 2 (17%), H-2→L + 1 (54%) | 212.9 | 0.006 | H-1→L + 2 (54%), HOMO→L + 3 (42%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, A.; Soliman, S.M.; Haukka, M.; Al-Majid, A.M.; Islam, M.S.; Ali, M.; Shaik, M.R. One-Pot Synthesis, X-ray Single Crystal and Molecular Insight of Enaminone-Based β-Morpholino-/N-Methylpiperazinyl-/Pyrrolidinylpropiophenone. Crystals 2020, 10, 282. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10040282
Barakat A, Soliman SM, Haukka M, Al-Majid AM, Islam MS, Ali M, Shaik MR. One-Pot Synthesis, X-ray Single Crystal and Molecular Insight of Enaminone-Based β-Morpholino-/N-Methylpiperazinyl-/Pyrrolidinylpropiophenone. Crystals. 2020; 10(4):282. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10040282
Chicago/Turabian StyleBarakat, Assem, Saied M. Soliman, Matti Haukka, Abdullah Mohammed Al-Majid, Mohammad Shahidul Islam, M. Ali, and Mohammed Rafi Shaik. 2020. "One-Pot Synthesis, X-ray Single Crystal and Molecular Insight of Enaminone-Based β-Morpholino-/N-Methylpiperazinyl-/Pyrrolidinylpropiophenone" Crystals 10, no. 4: 282. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10040282