Carbonate and Oxalate Crystallization by Interaction of Calcite Marble with Bacillus subtilis and Bacillus subtilis–Aspergillus niger Association
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial and Fungal Strains Identification
2.2. Experimental Conditions
2.3. X-Ray Powder Diffraction (XRD)
2.4. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDXS)
2.5. Determination of Biomass Content
2.6. Chromatography-Mass Spectrometry (GC–MS Analysis)
2.7. Determination of EPS Content
2.8. Determination of pH Values
3. Results
3.1. The Growth of Biomass on the Mineral Surface
3.2. The Metabolism of Bacillus subtilis and Bacillus subtilis with Aspergillus niger in the Cultural Liquids at Different Glucose Levels
3.2.1. Acid Production
3.2.2. The Extracellular Polymer Matrix Formation
3.2.3. Changes of pH Value
3.3. The Phase Composition of Synthesis Products and the Morphology of Crystals
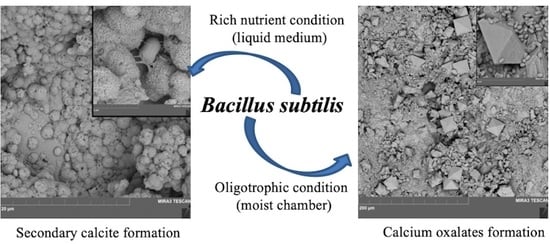
3.3.1. The Experiments with B. subtilis
Liquid Medium
Humidity Chamber
3.3.2. The experiments with B. subtilis and A. niger
Liquid Medium
Humidity Chamber
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boniek, D.; MendesIsolda, I.C.; Abreu, C.M.; Abreu, O.C.; Show, O.; Stoianoff, M.A.R. Ecology and identification of environmental fungi and metabolic processes involved in the biodeterioration of Brazilian soapstone historical monuments. Lett. Appl. Microbiol. 2017, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Gadd, G.M.; Bahri-Esfahani, J.; Li, Q.; Rhee, Y.J.; Wei, Z.; Fomina, M.; Liang, X. Oxalate production by fungi: Significance in geomycology, biodeterioration and bioremediation. Fungal Biol. Rev. 2014, 28, 36–55. [Google Scholar] [CrossRef]
- Sturm, E.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Zelenskaya, M.S.; Sazanova, K.V.; Rusakov, A.V.; Kniep, R. Crystallization of calcium oxalate hydrates by interaction of calcite marble with fungus Aspergillus niger. Am. Miner. 2015, 100, 2559–2565. [Google Scholar] [CrossRef]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Microbial deterioration of stone monuments—An updated overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar] [CrossRef]
- Savvides, A.L.; Nikolakopoulou, T.L.; Kyratsous, N.; Katsifas, E.A.; Kanini, G.; Karagoun, A.D. Bacterial Deterioration of Marble Monuments: A Case Study of the Conservation Project of Acropolis Monuments. Geomicrobiol. J. 2014, 31, 726–736. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, J.; Sun, H.; Huang, Y.; Tao, F.; Lian, B. Carbonate Mineral Formation under the Influence of Limestone-Colonizing Actinobacteria: Morphology and Polymorphism. Front. Microbiol. 2016, 7, 366. [Google Scholar] [CrossRef] [Green Version]
- Anbu, P.; Kang, C.-H.; Shin, Y.-J.; So, J.-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus 2016, 5, 250. [Google Scholar] [CrossRef] [Green Version]
- Muynck, W.D.; Belie, N.D.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Chen, L.; Xie, A.; Jia, R.; Shen, Y.; Tang, W.; Li, C. Influence of Bacillus subtilis on the growth of calcium oxalate. Cryst. Res. Technol. 2007, 42, 881–885. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Abbasi, R.R. Quantification of Exopolysaccharide Produced by Bacillus subtilis and the Effect of Different Factors on its Production. Raf. J. Sci. 2018, 27, 82–91. [Google Scholar]
- Monte, M. Oxalate film formation on marble specimens caused by fungus. J. Cult. Herit. 2003, 4, 255–258. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Vlasov, D.Y.; Osmolovskay, N.G.; Schiparev, S.M.; Rusakov, A.V. Significance and regulation of acids production by rock-inhabited fungi. In Biogenic—Abiogenic Interactions in Natural and Anthropogenic Systems. Lecture Notes in Earth System Sciences; Frank-Kamenetskaya, O., Panova, E., Vlasov, D., Eds.; Springer: Cham, Switzerland, 2016; pp. 379–392. [Google Scholar] [CrossRef]
- Keshari, N.; Adhikary, S.P. Characterization of cyanobacteria isolated from biofilms on stone monuments at Santiniketan, India. Biofouling 2013, 29, 525–536. [Google Scholar] [CrossRef]
- Gilbert, P.; Maira-Litran, T.; McBain, A.J.; Rickard, A.H.; Whyte, F.W. The physiology and collective recalcitrance of microbial biofilm communities. Adv. Microb. Physiol. 2002, 46, 203–256. [Google Scholar]
- Boonchan, S.; Britz, M.L.; Stanley, G.A. Degradation and mineralization of high-molecular weight polycyclic aromatic hydrocarbons by defined fungal-bacterial cocultures. Appl. Environ. Microbiol. 2000, 66, 1007–1019. [Google Scholar] [CrossRef] [Green Version]
- Berdoulay, M.; Salvado, J.C. Genetic characterization of microbial communities living at the surface of building stones. Lett. Appl. Microbiol. 2009, 49, 311–316. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A. A review of microbial precipitation for sustainable construction. Constr. Build. Mater. 2015, 93, 1224–1235. [Google Scholar] [CrossRef]
- Wang, R.; Qian, C. In situ restoration of the surface defects on cement-based materials by bacteria mineralization with spraying method. J. Wuhan Univ. Technol. Mat. Sci. Ed. 2014, 29, 518–526. [Google Scholar] [CrossRef]
- Dejong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Reeder, R.J.; Lamble, G.M.; Northrup, P.A. XAFS study of the coordination and local relaxation around Co2+, Zn2+, Pb2+, and Ba2+, trace elements in calcite. Am. Miner. 1999, 84, 1049–1060. [Google Scholar] [CrossRef]
- Kumari, D.; Quan, X.-Y.; Pan, X.; Achal, V.; Li, Q.; Gadd, G.M. Microbially-induced Carbonate Precipitation for Immobilization of Toxic Metals. Adv. Appl. Microbiol. 2016, 94, 79–108. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Huang, Z.; Li, X.; Liu, M.; Cheng, Y. Bio-remediation of acephate-Pb(II) Compound Contaminants by Bacillus Subtilis FZUL-33. J. Environ. Sci. 2016, 45, 94–99. [Google Scholar] [CrossRef]
- Rusakov, A.V.; Vlasov, A.D.; Zelenskaya, M.S.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y. The crystallization of calcium oxalate hydrates formed by interaction between microorganisms and minerals. In Biogenic—Abiogenic Interactions in Natural and Anthropogenic Systems. Lecture Notes in Earth System Sciences; Frank-Kamenetskaya, O., Panova, E., Vlasov, D., Eds.; Springer: Cham, Switzerland, 2016; pp. 357–377. [Google Scholar] [CrossRef]
- Castanier, S.Ł.; Métayer-Levrel, G.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis—The microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Sookie, S. Microbiological precipitation of CaCO3. Bang Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Wei, S.; Cui, H.; Jiang, Z.; Liu, H.; He, H.; Fang, N. Biomineralization processes of calcite induced by bacteria isolated from marine sediments. Braz. J. Microbiol. 2015, 46, 455–464. [Google Scholar] [CrossRef]
- Marvasi, M.; Gallagher, K.L.; Martinez, L.C.; Pagan, M.W.C.; Santiago, R.E.R.; Vega, G.C.; Vissche Pieter, T. Importance of B4 Medium in Determining Organomineralization Potential of Bacterial Environmental Isolates. Geomicrobiol. J. 2012, 29, 916–924. [Google Scholar] [CrossRef]
- Perito, M.B.; Buccianti, R.A.; Passaponti, M.; Montegrossi, G.; Di Benedetto, F. An XRPD and EPR spectroscopy study of microcrystalline calcite bioprecipitated by Bacillus subtilis. Phys. Chem. Miner. 2018, 45, 935–944. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, S.M.; Mukherjee, A. Biomineralization of Calcium Carbonate Polymorphs by the Bacterial Strains Isolated from Calcareous Sites. J. Microbiol. Biotechnol. 2013, 23, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, L.; Chen, W.; Yu, L.; Li, W.; Yu. H. Calcium carbonate precipitation and crystal morphology induced by microbial carbonic anhydrase and other biological factors. Process Biochem. 2010, 45, 1017–1021. [Google Scholar] [CrossRef]
- Ronholm, J.; Schumann, D.; Sapers, H.M.; Izawa, M.; Applin, D.; Berg, B.; Mann, P.; Vali, H.R.; Flemming, L.; Cloutis, E.A.; et al. Amineralogical characterization of biogenic calcium carbonates precipitated by heterotrophic bacteria isolated from cryophilic polar regions. Geobiology 2014, 12, 542–556. [Google Scholar] [CrossRef]
- Gorospe, C.M.; Han, S.-H.; Kim, S.-G.; Park, J.-Y.; Kang, C.-H.; Jeong, J.-H.; So, J.-S. Effects of Different Calcium Salts on Calcium Carbonate Crystal Formation by Sporosarcina pasteurii KCTC 3555. Biotechnol. Bioprocess Eng. 2013, 18, 903–908. [Google Scholar] [CrossRef]
- Somova, N.G.; Dobrovol’skaya, T.G.; Zenova, G.M.; Ivanovskii, R.N. Microbial growth of the masonry surface: Synecological analysis. Microbiology 1998, 67, 569–574. [Google Scholar]
- Kurakov, A.V.; Somova, N.G.; Ivanovskii, R.N. Micromycetes populating limestone and red brick surfaces of the Novodevichii Convent masonry. Microbiology 1999, 68, 232–241. [Google Scholar]
- Savadogo, A.C.; Ouattara, A.T.; Savadogo, P.W.; Barro, N.; Ouattara, A.S.; Traore, A.S. Identification of exopolysaccharides-producing lactic acid bacteria from Burkina Faso fermented milk samples. Afr. J. Biotechnol. 2004, 3, 189–194. [Google Scholar]
- Ghorbani, Y.; Oliazadeh, M.; Shahvedi, A.; Roohi, R.; Pirayehgar, A. Use of some isolated fungi in biological Leaching of Aluminum from low grade bauxite. Afr. J. Biotechnol. 2007, 6, 1284–1288. [Google Scholar]
- Maruthamuthu, S.; Dhandapani, P.; Ponmariappan, S.; Bae, J.-H.; Palaniswamy, N.; Rahman, P.K.S.M. Impact of ammonia producing Bacillus sp. on corrosion of cupronickel alloy 90:10. Met. Mater. Intl. 2009, 15, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Kuz’mina, M.A.; Rusakov, A.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y. The influence of inorganic and organic components of biofilms with microscopic fungi on the phase composition and morphology of crystallizing calcium oxalates. Cryst. Rep. 2019, 64, 161–167. [Google Scholar] [CrossRef]
- Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Rytikova, V.V. (Eds.) The Effect of the Environment on Saint Petersburg’s Cultural Heritage. Results of Monitoring the Historical Necropolis Monuments; Springer Nature Switzerland AG: Cham, Switzerland, 2019; 188p. [Google Scholar]






| Organism | Glucose Content, g/L | Days of Growth | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 14 | 21 | 30 | |||||||
| pH | Oxalic Acid, µg/mL | pH | Biomass, mg/100 mL | Oxalic Acid, µg/mL | EPS, µg/mL | pH | Oxalic Acid, µg/mL | ||
| Bacillus subtilis | 1 | 7.5 | not found | 7.8 | 0.33±0.01 | 1.0±0.4 | 328±44 | 8.2 | 1.1±0.3 |
| 5 | 7.5 | not found | 8.2 | 0.38±0.02 | 0.6±0.4 | 405±58 | 8.6 | 1.0±0.3 | |
| 10 | 7.0 | not found | 7.4 | 0.46±0.05 | 1.1±0.3 | 1121±181 | 8.0 | 1.5±0.3 | |
| 30 | 6.5 | not found | 7.2 | 0.678±0.01 | 0.8±0.1 | 1460±204 | 8.1 | 3.4±0.4 | |
| Bacillus subtilis +Aspergillus niger | 1 | 7.5 | 2.2±0.5 | 8.0 | 0.64±0.02 | 2.0±0.1 | 437±66 | 8.0 | 15.5±2.0 |
| 10 | 4.5 | 153.8±13.4 | 5.0 | 3.80±0.04 | 1032.9±89.1 | 1.1±0.2 | 7.0 | 1740.0±99.4 | |
| 30 | 3.0 | 740.0±20.8 | 5.5 | 4.11±0.08 | 3275.3±232.1 | 10±2 | 5.0 | 5275.1±100.4 | |
| Days of Experiment | Organisms | |||||||
|---|---|---|---|---|---|---|---|---|
| B. subtilis | B. subtilis + A. niger | |||||||
| Liquid Medium | ||||||||
| Glucose Concentration, g/L | ||||||||
| 1 | 10 | 30 | 1 | 10 | 30 | |||
| 14 | Marble dissolution | Separate clusters of small calcite globules, small content of whewellite, numerous intergrowths of brushite crystals | Strong marble dissolution | Single dipyramidal- prismatic weddellite crystals in a continuous carpet of plate whewellite crystals | ||||
| 21 | Marble dissolution | Separate clusters of small calcite globules | The beginning of calcite crust formation (thickness 5–10 μm), small content of whewellite, numerous intergrowths of brushite crystals | Separate clusters of small globules of secondary calcite, single dipyramidal weddellite and plate-like whewellite crystals. | Massive dipyramidal weddellite crystals in continuous carpet of plate whewellite crystals | Massive dipyramidal and dipyramidal- prismatic weddellite crystals and numerous whewellite spherulitic intergrowths | ||
| 30 | Separate clusters of small calcite globules, small content of the whewellite, numerous intergrowths of brushite crystals | The beginning of calcite crust formation (thickness 5–10 μm), small content of the whewellite, numerous intergrowths of brushite crystals | Continuous calcite crust (thickness up to 20 μm), small content of whewellite, numerous intergrowths of brushite crystals | Single dipyramidal- prismatic weddellite crystals in continuous carpet of plate whewellite crystals | Massive dipyramidal and dipyramidal–prismatic weddellite crystals and numerous whewellite spherulitic intergrowths | |||
| Humidity chamber | ||||||||
| 30 | Separate dipyramidal weddellite crystals | Separate dipyramidal–prismatic weddellite crystals | ||||||
| 60 | ||||||||
| 90 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sazanova, K.V.; Frank-Kamenetskaya, O.V.; Vlasov, D.Y.; Zelenskaya, M.S.; Vlasov, A.D.; Rusakov, A.V.; Petrova, M.A. Carbonate and Oxalate Crystallization by Interaction of Calcite Marble with Bacillus subtilis and Bacillus subtilis–Aspergillus niger Association. Crystals 2020, 10, 756. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10090756
Sazanova KV, Frank-Kamenetskaya OV, Vlasov DY, Zelenskaya MS, Vlasov AD, Rusakov AV, Petrova MA. Carbonate and Oxalate Crystallization by Interaction of Calcite Marble with Bacillus subtilis and Bacillus subtilis–Aspergillus niger Association. Crystals. 2020; 10(9):756. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10090756
Chicago/Turabian StyleSazanova (nee Barinova), Katerina V., Olga V. Frank-Kamenetskaya, Dmitry Yu. Vlasov, Marina S. Zelenskaya, Alexey D. Vlasov, Aleksei V. Rusakov, and Maya A. Petrova. 2020. "Carbonate and Oxalate Crystallization by Interaction of Calcite Marble with Bacillus subtilis and Bacillus subtilis–Aspergillus niger Association" Crystals 10, no. 9: 756. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst10090756