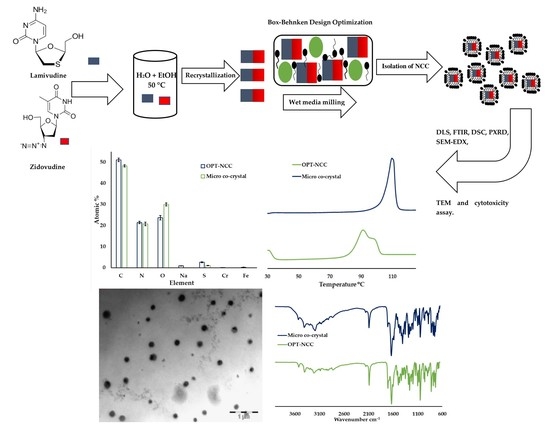
Top-Down Synthesis of a Lamivudine-Zidovudine Nano Co-Crystal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Co-Crystal Manufacture
2.2.2. NCC Manufacture
2.2.3. Optimisation of Electrosteric NCC (OPT-NCC).
2.2.4. Dynamic Light Scattering
2.2.5. Formulation Optimisation
2.2.6. Characterisation of Optimised NCC
FTIR Spectroscopy
Differential Scanning Calorimetry (DSC)
Powder X-ray Diffraction (PXRD)
Transmission Electron Microscopy (TEM)
Energy Dispersive X-ray Spectroscopy Scanning Electron Microscopy (SEM-EDX)
In Vitro Cytotoxicity
3. Results and Discussion
3.1. Formulation Development
3.1.1. Particle Size (PS)
3.1.2. Polydispersity Index (PDI)
3.1.3. Zeta Potential (ZP)
3.2. Formulation Optimisation
3.3. Characterisation of OPT-NCC
3.3.1. FTIR
3.3.2. DSC
3.3.3. PXRD
3.3.4. TEM
3.3.5. SEM-EDX
3.3.6. In Vitro Cytotoxicity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- 1UNAIDS UNAIDS Data; 2019. Available online: https://www.unaids.org/en/resources/documents/2019/2019-UNAIDS-data (accessed on 29 December 2020).
- 2UNAIDS UNAIDS Data; 2020. Available online: https://www.unaids.org/en/resources/documents/2020/unaids-data (accessed on 29 December 2020).
- World Health Organization. National Centre for AIDS and STD Control National Anti-Retroviral Therapy Guidelines; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Cihlar, T.; Ray, A.S. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antivir. Res. 2010, 85, 39–58. [Google Scholar] [CrossRef]
- Perry, C.M.; Faulds, D. Lamivudine. Drugs 1997, 53, 657–680. [Google Scholar] [CrossRef]
- Mandelbrot, L.; Landreau-Mascaro, A.; Rekacewicz, C.; Berrebi, A.; Benifla, J.L.; Burgard, M.; Lachassine, E.; Barret, B.; Chaix, M.-L.; Bongain, A.; et al. Lamivudine-Zidovudine Combination for Prevention of Maternal-Infant Transmission of HIV-1. Obstet. Gynecol. Surv. 2001, 56, 603–604. [Google Scholar] [CrossRef]
- Govender, T.; Ojewole, E.; Naidoo, P.; Mackraj, I. Polymeric Nanoparticles for Enhancing Antiretroviral Drug Therapy. Drug Deliv. 2008, 15, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Flexner, C. Antiretroviral Agents and Treatment of HIV infection. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Carr, A.; Cooper, D.A. Adverse effects of antiretroviral therapy. Lancet 2000, 356, 1423–1430. [Google Scholar] [CrossRef]
- Johnson, M.A.; Moore, K.H.; Yuen, G.J.; Bye, A.; Pakes, G.E. Clinical Pharmacokinetics of Lamivudine. Clin. Pharmacokinet. 1999, 36, 41–66. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Peterson, M.L.; Hickey, M.B.; Zhang, Z.; Almarsson, Ö. Expanding the scope of crystal form evaluation in pharmaceutical science. J. Pharm. Pharm. Sci. 2006, 9, 317–326. [Google Scholar]
- Sander, J.R.G.; Bučar, D.-K.; Henry, R.F.; Zhang, G.G.Z.; MacGillivray, L.R. Pharmaceutical Nano-Cocrystals: Sonochemical Synthesis by Solvent Selection and Use of a Surfactant. Angew. Chem. Int. Ed. 2010, 49, 7284–7288. [Google Scholar] [CrossRef]
- Maginn, S. Crystal engineering: The design of organic solidsby G. R. Desiraju. J. Appl. Crystallogr. 1991, 24, 265. [Google Scholar] [CrossRef] [Green Version]
- Karashima, M.; Kimoto, K.; Yamamoto, K.; Kojima, T.; Ikeda, Y. A novel solubilization technique for poorly soluble drugs through the integration of nanocrystal and cocrystal technologies. Eur. J. Pharm. Biopharm. 2016, 107, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef]
- De Waard, H.; Frijlink, H.W.; Hinrichs, W.L. Bottom-Up Preparation Techniques for Nanocrystals of Lipophilic Drugs. Pharm. Res. 2010, 28, 1220–1223. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yaragudi, N.; Afolabi, A.; Dave, R.; Bilgili, E. Sub-100nm drug particle suspensions prepared via wet milling with low bead contamination through novel process intensification. Chem. Eng. Sci. 2015, 130, 207–220. [Google Scholar] [CrossRef]
- Juhnke, M.; Martin, D.; John, E. Generation of wear during the production of drug nanosuspensions by wet media milling. Eur. J. Pharm. Biopharm. 2012, 81, 214–222. [Google Scholar] [CrossRef]
- Juhnke, M.; Berghausen, J.; Timpe, C. Accelerated Formulation Development for Nanomilled Active Pharmaceutical Ingredients Using a Screening Approach. Chem. Eng. Technol. 2010, 33, 1412–1418. [Google Scholar] [CrossRef]
- Liu, P.; de Wulf, O.; Laru, J.; Heikkilä, T.; van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Peltonen, L.; Laaksonen, T. Dissolution Studies of Poorly Soluble Drug Nanosuspensions in Non-sink Conditions. AAPS PharmSciTech 2013, 14, 748–756. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Azad, M.; Davé, R.; Bilgili, E. Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective. Pharmaceutics 2016, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Becker, M.; Schwedes, J. Comminution of ceramics in stirred media mills and wear of grinding beads. Powder Technol. 1999, 105, 374–381. [Google Scholar] [CrossRef]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Miconazole nanosuspensions: Influence of formulation variables on particle size reduction and physical stability. Int. J. Pharm. 2010, 396, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kwade, A. Wet comminution in stirred media mills—Research and its practical application. Powder Technol. 1999, 105, 14–20. [Google Scholar] [CrossRef]
- Brosh, T.; Kalman, H.; Levy, A.; Peyron, I.; Ricard, F. DEM–CFD simulation of particle comminution in jet-mill. Powder Technol. 2014, 257, 104–112. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; McGurk, S.L.; Liversidge, G.G. Insulin Nanoparticles: A Novel Formulation Approach for Poorly Water-Soluble Zn-Insulin. Pharm. Res. 2004, 21, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.M.; York, P.; Ali, A.M.A.; Blagden, N. Hydrocortisone nanosuspensions for ophthalmic delivery: A comparative study between microfluidic nanoprecipitation and wet milling. J. Control. Release 2011, 149, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Orthmann, S.; Bellini, M.; Staufenbiel, S.; Bodmeier, R. Influence of Drug Brittleness, Nanomilling Time, and Freeze-Drying on the Crystallinity of Poorly Water-Soluble Drugs and Its Implications for Solubility Enhancement. AAPS PharmSciTech 2017, 18, 2437–2445. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef]
- Chamarthy, S.P.; Pinal, R. The nature of crystal disorder in milled pharmaceutical materials. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 331, 68–75. [Google Scholar] [CrossRef]
- Crowley, K.J.; Zografi, G. Cryogenic grinding of indomethacin polymorphs and solvates: Assessment of amorphous phase formation and amorphous phase physical stability. J. Pharm. Sci. 2002, 91, 492–507. [Google Scholar] [CrossRef]
- Sharma, P.; Denny, W.A.; Garg, S. Effect of wet milling process on the solid state of indomethacin and simvastatin. Int. J. Pharm. 2009, 380, 40–48. [Google Scholar] [CrossRef] [PubMed]
- De Smet, L.; Saerens, L.; de Beer, T.; Carleer, R.; Adriaensens, P.; van Bocxlaer, J.; Vervaet, C.; Remon, J.P. Formulation of itraconazole nanococrystals and evaluation of their bioavailability in dogs. Eur. J. Pharm. Biopharm. 2014, 87, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, J.; Wang, S.; Li, W.; Kebebe, D.; Zhang, Y.; Qi, D.; Guo, P.; Li, N.; Zhidong, L. A nano-cocrystal strategy to improve the dissolution rate and oral bioavailability of baicalein. Asian J. Pharm. Sci. 2019, 14, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Witika, B.; Mweetwa, L.; Matafwali, S.; Chabalenge, B.; Mwila, C.; Kalungia, A.C.; Nkanga, C.I.; Bapolisi, A.M.; Walker, R. Biocompatibility of Biomaterials for Nanoencapsulation: Current Approaches. Nanomaterilas 2020, 10, 1649. [Google Scholar] [CrossRef] [PubMed]
- Nielloud, F.; Marti-Mestres, G. Pharmaceutical Emulsions and Suspensions; Informa UK Limited: London, UK, 2000. [Google Scholar]
- Eon-Duval, A.; Valax, P.; Solacroup, T.; Broly, H.; Gleixner, R.; E Strat, C.L.; Sutter, J. Application of the quality by design approach to the drug substance manufacturing process of an Fc fusion protein: Towards a global multi-step design space. J. Pharm. Sci. 2012, 101, 3604–3618. [Google Scholar] [CrossRef]
- Martin-Moe, S.; Lim, F.J.; Wong, R.L.; Alavattam, S.; Sundaram, J.; Sane, S.U. A new roadmap for biopharmaceutical drug product development: Integrating development, validation, and quality by design. J. Pharm. Sci. 2011, 100, 3031–3043. [Google Scholar] [CrossRef]
- Bhatia, H.; Read, E.; Agarabi, C.D.; Brorson, K.; Lute, S.; Yoon, S. A design space exploration for control of Critical Quality Attributes of mAb. Int. J. Pharm. 2016, 512, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Qiao, Y.; Wu, Z. Nanosystem trends in drug delivery using quality-by-design concept. J. Control. Release 2017, 256, 9–18. [Google Scholar] [CrossRef]
- Nowacek, A.S.; McMillan, J.; Miller, R.; Anderson, A.; Rabinow, B.; Gendelman, H.E. Nanoformulated Antiretroviral Drug Combinations Extend Drug Release and Antiretroviral Responses in HIV-1-Infected Macrophages: Implications for NeuroAIDS Therapeutics. J. Neuroimmune Pharmacol. 2010, 5, 592–601. [Google Scholar] [CrossRef] [Green Version]
- Orenstein, J.M. Replication of HIV-1 in Vivo and in Vitro. Ultrastruct. Pathol. 2007, 31, 151–167. [Google Scholar] [CrossRef]
- Koppensteiner, H.; Brack-Werner, R.; Schindler, M. Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology 2012, 9, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van’t Klooster, G.; Hoeben, E.; Borghys, H.; Looszova, A.; Bouche, M.-P.; van Velsen, F.; Baert, L. Pharmacokinetics and Disposition of Rilpivirine (TMC278) Nanosuspension as a Long-Acting Injectable Antiretroviral Formulation. Antimicrob. Agents Chemother. 2010, 54, 2042–2050. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.; Jefferies, C.; Cryan, S.-A. Targeted Liposomal Drug Delivery to Monocytes and Macrophages. J. Drug Deliv. 2011, 2011, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.; Schfer, V.; Steffan, A.; Royer, C.; Kreuter, J.; Rübsamen-Waigmann, H.; von Briesen, H.; Schäfer, V. Inhibition of HIV in vitro by antiviral drug-targeting using nanoparticles. Res. Virol. 1994, 145, 215–220. [Google Scholar] [CrossRef]
- Désormeaux, A.; Bergeron, M.G. Lymphoid Tissue Targeting of Anti-HIV Drugs Using Liposomes. Methods Enzymol. 2005, 391, 330–351. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, F. Targeting to macrophages: Role of physicochemical properties of particulate carriers—Liposomes and microspheres—On the phagocytosis by macrophages. J. Control. Release 2002, 79, 29–40. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Uptake characteristics of liposomes by rat alveolar macrophages: Influence of particle size and surface mannose modification. J. Pharm. Pharmacol. 2007, 59, 75–80. [Google Scholar] [CrossRef]
- Chono, S.; Tauchi, Y.; Morimoto, K. Influence of Particle Size on the Distributions of Liposomes to Atherosclerotic Lesions in Mice. Drug Dev. Ind. Pharm. 2006, 32, 125–135. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Influence of particle size on drug delivery to rat alveolar macrophages following pulmonary administration of ciprofloxacin incorporated into liposomes. J. Drug Target. 2006, 14, 557–566. [Google Scholar] [CrossRef]
- Baert, L.; Klooster, G.V.T.; Dries, W.; François, M.; Wouters, A.; Basstanie, E.; Iterbeke, K.; Stappers, F.; Stevens, P.; Schueller, L.; et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur. J. Pharm. Biopharm. 2009, 72, 502–508. [Google Scholar] [CrossRef]
- Witika, B.; Smith, V.J.; Walker, R. Quality by Design Optimization of Cold Sonochemical Synthesis of Zidovudine-Lamivudine Nanosuspensions. Pharmaceutics 2020, 12, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witika, B.; Smith, V.J.; Walker, R. A Comparative Study of the Effect of Different Stabilizers on the Critical Quality Attributes of Self-Assembling Nano Co-Crystals. Pharmaceutics 2020, 12, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Chattopadhyay, N.; Wu, X.Y.; Bendayan, R. Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Adv. Drug Deliv. Rev. 2010, 62, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Toziopoulou, F.; Malamatari, M.; Nikolakakis, I.; Kachrimanis, K. Production of aprepitant nanocrystals by wet media milling and subsequent solidification. Int. J. Pharm. 2017, 533, 324–334. [Google Scholar] [CrossRef]
- Peltonen, L. Design Space and QbD Approach for Production of Drug Nanocrystals by Wet Media Milling Techniques. Pharmaceutics 2018, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Srivalli, K.M.R.; Mishra, B. Drug nanocrystals: A way toward scale-up. Saudi Pharm. J. 2016, 24, 386–404. [Google Scholar] [CrossRef] [Green Version]
- Ige, P.P.; Baria, R.K.; Gattani, S.G. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Coll. Surf. B Biointerf. 2013, 108, 366–373. [Google Scholar] [CrossRef]
- Wigger-Alberti, W.; Krebs, A.; Elsner, P. Experimental irritant contact dermatitis due to cumulative epicutaneous exposure to sodium lauryl sulphate and toluene: Single and concurrent application. Br. J. Dermatol. 2000, 143, 551–556. [Google Scholar] [CrossRef]
- Adair, J.H.; Suvaci, E.; Sindel, J. Surface and Colloid Chemistry. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P.B.T.-E., Eds.; Elsevier: Oxford, UK, 2001; pp. 1–10. ISBN 978-0-08-043152-9. [Google Scholar]
- Keck, C.M. Cyclosporine Nanosuspensions: Optimised Size Characterisation and Oral Formulations. Ph.D. Thesis, Freie Universitat Berlin, Berlin, Germany, 2006. [Google Scholar]
- Lopeandía, A.; Rodríguez-Viejo, J. Size-dependent melting and supercooling of Ge nanoparticles embedded in a SiO2 thin film. Thermochim. Acta 2007, 461, 82–87. [Google Scholar] [CrossRef]
- Sun, J.; Simon, S.L. The melting behavior of aluminum nanoparticles. Thermochim. Acta 2007, 463, 32–40. [Google Scholar] [CrossRef]
- Diwan, B.D. Size Effect on the Cohesive Energy of Palladium Nanoparticle. J. Comput. Theor. Nanosci. 2013, 10, 2779–2781. [Google Scholar] [CrossRef]
- Borchert, H.; Shevchenko, E.V.; Robert, A.; Mekis, I.; Kornowski, A.; Grübel, A.G.; Weller, H. Determination of Nanocrystal Sizes: A Comparison of TEM, SAXS, and XRD Studies of Highly Monodisperse CoPt3Particles. Langmuir 2005, 21, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, R.; Li, J.; Xu, L. A Comparative Study of Particle Size Dependency of IR and XRD Methods for Quartz Analysis. Am. Ind. Hyg. Assoc. J. 1994, 55, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Lang, A. X-ray diffraction procedures for polycrystal-line and amorphous materials. Acta Met. 1956, 4, 102. [Google Scholar] [CrossRef]
- Kamb, W.B. Theory of Preferred Crystal Orientation Developed by Crystallization under Stress. J. Geol. 2009, 67, 153–170. [Google Scholar] [CrossRef]
- Li, X.-S.; Wang, J.-X.; Shen, Z.-G.; Zhang, P.-Y.; Chen, J.-F.; Yun, J. Preparation of uniform prednisolone microcrystals by a controlled microprecipitation method. Int. J. Pharm. 2007, 342, 26–32. [Google Scholar] [CrossRef]
- Hennart, S.; Domingues, M.; Wildeboer, W.; van Hee, P.; Meesters, G. Study of the process of stirred ball milling of poorly water-soluble organic products using factorial design. Powder Technol. 2010, 198, 56–60. [Google Scholar] [CrossRef]
- Romberg, B.; Hennink, W.E.; Storm, G. Sheddable Coatings for Long-Circulating Nanoparticles. Pharm. Res. 2007, 25, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, P.; Bothiraja, C.; Pawar, A.P. Methotrexate-Loaded Nanomixed Micelles: Formulation, Characterization, Bioavailability, Safety, and In Vitro Anticancer Study. J. Pharm. Innov. 2018, 13, 213–225. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]








| Std Run | Formulation Code | No. of Balls (A) | Milling Time min (B) | SLS * % w/v (C) | TPGS 1000 * % w/v (D) |
|---|---|---|---|---|---|
| 4 | 1 | 3 | 30 | 0.5 | 1.5 |
| 6 | 2 | 2 | 20 | 1.0 | 1.0 |
| 21 | 3 | 2 | 10 | 0.5 | 1.0 |
| 9 | 4 | 1 | 20 | 0.5 | 1.0 |
| 24 | 5 | 2 | 30 | 0.5 | 2.0 |
| 12 | 6 | 3 | 20 | 0.5 | 2.0 |
| 13 | 7 | 2 | 10 | 0.0 | 1.5 |
| 10 | 8 | 3 | 20 | 0.5 | 1.0 |
| 19 | 9 | 1 | 20 | 1.0 | 1.5 |
| 17 | 10 | 1 | 20 | 0.0 | 1.5 |
| 20 | 11 | 3 | 20 | 1.0 | 1.5 |
| 7 | 12 | 2 | 20 | 0.0 | 2.0 |
| 26 | 13 | 2 | 20 | 0.5 | 1.5 |
| 18 | 14 | 3 | 20 | 0.0 | 1.5 |
| 23 | 15 | 2 | 10 | 0.5 | 2.0 |
| 15 | 16 | 2 | 10 | 1.0 | 1.5 |
| 25 | 17 | 2 | 20 | 0.5 | 1.5 |
| 11 | 18 | 1 | 20 | 0.5 | 2.0 |
| 8 | 19 | 2 | 20 | 1.0 | 2.0 |
| 5 | 20 | 2 | 20 | 0.0 | 1.0 |
| 28 | 21 | 2 | 20 | 0.5 | 1.5 |
| 14 | 22 | 2 | 30 | 0.0 | 1.5 |
| 2 | 23 | 3 | 10 | 0.5 | 1.5 |
| 22 | 24 | 2 | 30 | 0.5 | 1.0 |
| 3 | 25 | 1 | 30 | 0.5 | 1.5 |
| 29 | 26 | 2 | 20 | 0.5 | 1.5 |
| 16 | 27 | 2 | 30 | 1.0 | 1.5 |
| 27 | 28 | 2 | 20 | 0.5 | 1.5 |
| 1 | 29 | 1 | 10 | 0.5 | 1.5 |
| Number of Balls | Milling Time min | SLS Conc. %w/v | TPGS Conc. %w/v | D |
|---|---|---|---|---|
| 2 | 30 | 1 | 1.5 | 1 |
| Std. Run | Formulation Code | * PS nm | * PDI | * ZP mV |
|---|---|---|---|---|
| 4 | 1 | 456.1 ± 86.9 | 0.504 ± 0.113 | −28.7 ± 1.2 |
| 6 | 2 | 455.5 ± 16.5 | 0.513 ± 0.070 | −43.6 ± 3.9 |
| 21 | 3 | 548.7 ± 25.5 | 0.49 ± 0.023 | −28.5 ± 2.4 |
| 9 | 4 | 836.1 ± 14.7 | 0.537 ± 0.029 | −29.7 ± 1.8 |
| 24 | 5 | 553.8 ± 26.9 | 0.556 ± 0.023 | −20.4 ± 0.6 |
| 12 | 6 | 486.5 ± 38.2 | 0.516 ± 0. 026 | −19.4 ± 0.7 |
| 13 | 7 | 216.1 ± 22.1 | 0.364 ± 0.092 | −8.4 ± 0.1 |
| 10 | 8 | 194.5 ± 58.6 | 0.414 ± 0.108 | −28.4 ± 1.2 |
| 19 | 9 | 229.5 ± 33.7 | 0.479 ± 0.099 | −42.8 ± 4.6 |
| 17 | 10 | 1167 ± 29.0 | 0.495 ± 0.020 | −8.2 ± 0.7 |
| 20 | 11 | 148.0 ± 17.7 | 0.434 ± 0.011 | −49 ± 3.2 |
| 7 | 12 | 893.7 ± 40.4 | 0.517 ± 0.180 | −8.3 ± 0.5 |
| 26 | 13 | 205.3 ± 2.8 | 0.557 ± 0.041 | −27.9 ± 2.3 |
| 18 | 14 | 1643 ± 28.5 | 0.224 ± 0.090 | −6.2 ± 1.1 |
| 23 | 15 | 315.7 ± 40.2 | 0.507 ± 0.111 | −27.7 ± 2.9 |
| 15 | 16 | 219.1 ± 28.9 | 0.415 ± 0,060 | −43.3 ± 3.2 |
| 25 | 17 | 461.4 ± 47.2 | 0.37 ± 0.021 | −29.9 ± 1.2 |
| 11 | 18 | 692.1 ± 88.2 | 0.576 ± 0.047 | −29.3 ± 2.7 |
| 8 | 19 | 153.3 ± 28.8 | 0.51 ± 0.087 | −31.8 ± 3.1 |
| 5 | 20 | 625.7 ± 48.2 | 0.307 ± 0.077 | −13.7 ± 1.2 |
| 28 | 21 | 235.3 ± 22.7 | 0.428 ± 0.049 | −30.1 ± 1.1 |
| 14 | 22 | 840.6 ± 83.7 | 0.444 ± 0.035 | −27.1 ± 22.6 |
| 2 | 23 | 783.1 ± 76.2 | 0.761 ± 0.045 | −29 ± 2.1 |
| 22 | 24 | 278.8 ±52.3 | 0.355 ± 0.055 | −28.5 ± 3.0 |
| 3 | 25 | 1042 ± 18.4 | 0.355 ± 0.152 | −26.6 ± 2.1 |
| 29 | 26 | 305.7 ± 62.8 | 0.497 ± 0.076 | −29.8 ± 0.9 |
| 16 | 27 | 215.6 ± 37.7 | 0.364 ± 0.143 | −42.3 ± 0.3 |
| 27 | 28 | 239.1 ± 48.2 | 0.419 ± 0.106 | −30.2 ± 0.8 |
| 1 | 29 | 498.2 ± 31.3 | 0.464 ± 0.037 | −30.4 ± 1.8 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 14 | 1.91 × 105 | 3.02 | 0.0237 | ||
| A: Number of balls | 47,338.6 | 1 | 47,338.64 | 0.75 | 0.4019 | |
| B: Milling Time | 54,136.3 | 1 | 54,136.33 | 0.85 | 0.3709 | |
| C: SLS conc. | 1.31 × 106 | 1 | 1.31 × 106 | 20.69 | 0.0005 | |
| D: TPGS 1000 conc. | 2022.8 | 1 | 2022.8 | 0.032 | 0.8607 | |
| AB | 1.90 × 105 | 1 | 1.90 × 105 | 2.99 | 0.1056 | |
| AC | 77,701.6 | 1 | 77,701.56 | 1.23 | 0.2867 | |
| AD | 47,524 | 1 | 47,524 | 0.75 | 0.401 | |
| BC | 98,596 | 1 | 98,596 | 1.56 | 0.2326 | |
| BD | 64,516 | 1 | 64,516 | 1.02 | 0.33 | |
| CD | 81,282 | 1 | 81,282.01 | 1.28 | 0.2763 | |
| A2 | 6.44 × 105 | 1 | 6.44 × 105 | 10.17 | 0.0066 | |
| B2 | 9916.18 | 1 | 9916.18 | 0.16 | 0.6983 | |
| C2 | 1.35 × 105 | 1 | 1.35 × 105 | 2.12 | 0.1671 | |
| D2 | 14,588.4 | 1 | 14,588.44 | 0.23 | 0.6387 | |
| Residual | 8.867 × 105 | 14 | 63,338.53 | |||
| Lack of Fit | 8.444 × 105 | 10 | 84,436.01 | 7.97 | 0.0301 | |
| Pure Error | 42,379.4 | 4 | 10,594.85 | |||
| Cor Total | 28 | |||||
| Source | Sum of Squares | Df | Mean Square | F-Value | P-Value Prob > F |
|---|---|---|---|---|---|
| Model | 0.053 | 4 | 0.013 | 1.37 | 0.2724 |
| A: Number of balls | 2.341 × 10−4 | 1 | 2.34 × 10−4 | 0.024 | 0.8773 |
| B:Milling Time | 0.015 | 1 | 0.015 | 1.55 | 0.2251 |
| C: SLS conc. | 0.011 | 1 | 0.011 | 1.15 | 0.2947 |
| D: TPGS 1000 conc. | 0.027 | 1 | 0.027 | 2.78 | 0.1087 |
| Residual | 0.23 | 24 | 9.62 × 10−3 | ||
| Lack of Fit | 0.21 | 20 | 0.01 | 1.96 | 0.2718 |
| Pure Error | 0.021 | 4 | 5.35 × 10−3 | ||
| Cor Total | 0.28 | 28 | |||
| Source | Sum of Squares | Df | Mean Square | F Value | P-Value Prob > F |
|---|---|---|---|---|---|
| Model | 3990.57 | 4 | 997.64 | 45.51 | <0.0001 |
| A: Number of balls | 3.31 | 1 | 3.31 | 0.15 | 0.7011 |
| B: Milling Time | 12.61 | 1 | 12.61 | 0.58 | 0.4556 |
| C: SLS conc. | 3920.47 | 1 | 3920.47 | 178.85 | <0.0001 |
| D: TPGS 1000 conc. | 54.19 | 1 | 54.19 | 2.47 | 0.129 |
| Residual | 526.09 | 24 | 21.92 | ||
| Lack of Fit | 522.46 | 20 | 26.12 | 28.8 | 0.0025 |
| Pure Error | 3.63 | 4 | 0.91 | ||
| Cor Total | 4516.66 | 28 |
| Response | Predicted | Experimental | % Predicted Error |
|---|---|---|---|
| PS (nm) | 252.18 | 271.0 ± 92.0 | 7.47 |
| PDI | 0.456 | 0.467 ± 0.073 | 2.41 |
| ZP (mV) | −47.9 | −41.9 ± 3.9 | 12.53 |
| Element | OPT-NCC | Micro Co-Crystal |
|---|---|---|
| Atomic % | Atomic % | |
| C | 51.07 ± 0.88 | 48.26 ± 0.52 |
| N | 21.43 ± 0.62 | 20.75 ± 0.87 |
| O | 23.70 ± 1.06 | 29.96 ± 0.73 |
| Na | 0.90 ± 0.04 | - |
| S | 2.63 ± 0.21 | 1.03 ± 0.09 |
| Cr | 0.06 ± 0.01 | - |
| Fe | 0.21 ± 0.08 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witika, B.A.; Smith, V.J.; Walker, R.B. Top-Down Synthesis of a Lamivudine-Zidovudine Nano Co-Crystal. Crystals 2021, 11, 33. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11010033
Witika BA, Smith VJ, Walker RB. Top-Down Synthesis of a Lamivudine-Zidovudine Nano Co-Crystal. Crystals. 2021; 11(1):33. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11010033
Chicago/Turabian StyleWitika, Bwalya A., Vincent J. Smith, and Roderick B. Walker. 2021. "Top-Down Synthesis of a Lamivudine-Zidovudine Nano Co-Crystal" Crystals 11, no. 1: 33. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11010033