Synthesis, Photophysical, and Computational Studies of a Bridged IrΙΙΙ-PtΙΙ Heterodimetallic Complex
Abstract
:1. Introduction
2. Experimental Section
2.1. Spectroscopic Analysis
2.2. Emission Lifetime Analysis
2.3. DFT and TDDFT Calculations
2.4. X-ray Crystallography
2.5. Synthesis
2.5.1. [(dapz)PtCl2] (2)
2.5.2. [(. ppy)2Ir(dapz)]Cl (3)
2.5.3. [(. ppy)2Ir(dapz)PtCl2]Cl (4)
3. Results and Discussion
3.1. Synthesis and Single Crystal X-ray Analysis
3.2. DFT Calculations
3.3. Absorption Studies and TDDFT Calculations
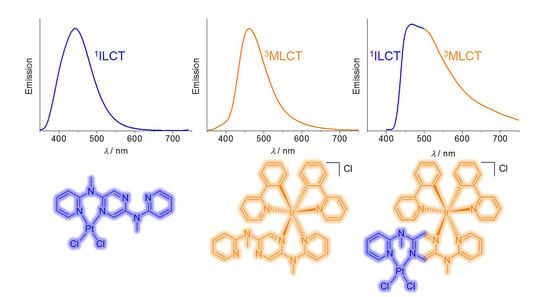
3.4. Emission Spectroscopic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Magde, D.; Magde, M.D., Jr.; Glazer, E.C. So-called “dual emission” for 3MLCT luminescence in ruthenium complex ions: What is really happening? Coord. Chem. Rev. 2016, 306, 447–467. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.-Y.; Wu, S.-H.; Yang, R.; Zhong, Y.-W.; Gong, Z.-L. Dual-emissive transition-metal complexes and their applications as ratiometric photoluminescent probes. Sci. Sin. Chim. 2020, 50, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Keyes, T.E.; O’Connor, C.; Vos, J.G. Evidence for the presence of dual emission in a ruthenium(II) polypyridyl mixed ligand complex. Chem. Commun. 1998, 1, 889–890. [Google Scholar] [CrossRef]
- Zambrana, J.L.; Ferloni, E.X.; Colis, J.C.; Gafney, H.D. Multiple Charge-Transfer Emissions from Different Metal-Ligand Pairs in Ruthenium Diimines. Inorg. Chem. 2008, 47, 2–4. [Google Scholar] [CrossRef]
- Song, L.; Feng, J.; Wang, X.; Yu, J.; Hou, Y.; Xie, P.; Zhang, B.; Xiang, J.; Ai, X.; Zhang, J. Dual Emission from 3MLCT and 3ILCT Excited States in a New Ru(II) Diimine Complex. Inorg. Chem. 2003, 42, 3393–3395. [Google Scholar] [CrossRef] [PubMed]
- Glazer, E.C.; Magde, A.D.; Tor, Y. Ruthenium Complexes That Break the Rules: Structural Features Controlling Dual Emission. J. Am. Chem. Soc. 2007, 129, 8544–8551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blakley, R.L.; Myrick, M.L.; Dearmond, M.K. Interligand and Charge-Transfer Emission from [Ru(bpy)(HDPA)2]2+: A Du-al-Emitting Ru(II) Complex. J. Am. Chem. Soc. 1986, 108, 7843–7844. [Google Scholar] [CrossRef]
- King, K.A.; Watts, R.J. ChemInform Abstract: Dual Emission from an Ortho-Metalated Ir(III) Complex. Cheminform 1987, 18, 1589–1590. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, N.; Li, Y.; Zhao, W.; Quan, Y.; Cheng, Y.; Chen, H.Y.; Xu, J.J. Trace Ir(III) complex enhanced electrochemiluminescence of AIE-active Pdots in aqueous media. Sci. China Chem. 2020, 63, 715–721. [Google Scholar] [CrossRef]
- Scattergood, P.A.; Ranieri, A.M.; Charalambou, L.; Comia, A.; Ross, D.A.W.; Rice, C.R.; Hardman, S.J.O.; Heully, J.L.; Dixon, I.M.; Massi, M.; et al. Unravelling the Mechanism of Excited-State Interligand Energy Transfer and the En-gineering of Dual Emission in [Ir(C∧N)2(N∧N)]+ Complexes. Inorg. Chem. 2020, 59, 1785–1803. [Google Scholar] [CrossRef]
- Cao, Y.; Wolf, M.O.; Patrick, B.O. Dual-Emissive Platinum(II) Metallacycles with Thiophene-Containing Bisacetylide Ligands. Inorg. Chem. 2016, 55, 8985–8993. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Scholz, N.; Behnke, T.; Resch-Genger, U.; Heinze, K. Thermo-Chromium: A Contactless Optical Molecular Ther-mometer. Chem. Eur. J. 2017, 23, 12131–12135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.-M.; Yeh, Y.-S.; Ho, M.-L.; Chou, P.-T.; Chen, P.-S.; Chi, Y. Dual Room-Temperature Fluorescent and Phosphorescent Emission in 8-Quinolinolate Osmium(II) Carbonyl Complexes: Rationalization and Generalization of Intersystem Crossing Dynamics. Inorg. Chem. 2005, 44, 4594–4603. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Zhao, Z.; Li, X.; Yu, X.; Huo, P.; Jin, Q.; Liu, Z.; Bian, Z.; Huang, C. Two-Coordinate Copper(I)/NHC Com-plexes: Dual Emission Properties and Ultralong Room-Temperature Phosphorescence. Angew. Chem. Int. Ed. 2020, 59, 8210–8217. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Han, Y.; Lee, Y.-M.; Park, S.Y.; Nam, W.; Lippard, S.J. Phosphorescent Sensor for Robust Quantification of Copper(II) Ion. J. Am. Chem. Soc. 2011, 133, 11488–11491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Guo, H.; Zhao, J. Ratiometric luminescent molecular oxygen sensors based on uni-luminophores of C⁁N Pt(ii)(acac) complexes that show intense visible-light absorption and balanced fluorescence/phosphorescence dual emission. Chem. Commun. 2011, 47, 11471–11473. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Byrne, A.; Dolan, C.; Forster, R.J.; Keyes, T.E. Solvent switchable dual emission from a bichromophoric ruthenium–BODIPY complex. Chem. Commun. 2015, 51, 15839–15841. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, X.; Cao, T.; Zhang, K.Y.; Yang, L.; Liu, S.; Liang, H.; Yang, H.; Li, F.; Huang, W. Fluorescent/phosphorescent dual-emissive conjugated polymer dots for hypoxia bioimaging. Chem. Sci. 2015, 6, 1825–1831. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.Y.; Gao, P.; Sun, G.; Zhang, T.; Li, X.; Liu, S.; Zhao, Q.; Lo, K.K.-W.; Huang, W. Dual-Phosphorescent Iridium(III) Complexes Extending Oxygen Sensing from Hypoxia to Hyperoxia. J. Am. Chem. Soc. 2018, 140, 7827–7834. [Google Scholar] [CrossRef]
- Gupta, S.K.; Haridas, A.; Choudhury, J. Remote Terpyridine Integrated NHC–IrIII Luminophores as Potential Dual-Emissive Ratiometric O2 Probes. Chem. Eur. J. 2017, 23, 4770–4773. [Google Scholar] [CrossRef]
- Lo, K.K.-W.; Zhang, K.Y.; Leung, S.-K.; Tang, M.-C. Exploitation of the Dual-emissive Properties of Cyclometalated Iridium(III)–Polypyridine Complexes in the Development of Luminescent Biological Probes. Angew. Chem. Int. Ed. 2008, 47, 2213–2216. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-Y.; Wu, S.-H.; Ma, J.; Gong, Z.-L.; Sun, T.-G.; Jin, Y.; Yang, R.; Sun, B.; Zhong, Y.-W. Ratiometric detection of amyloid-β aggregation by a dual-emissive tris-heteroleptic ruthenium complex. Chem. Commun. 2020, 56, 2087–2090. [Google Scholar] [CrossRef]
- Walker, M.G.; Ramu, V.; Meijer, A.J.H.M.; Das, A.; Thomas, J.A. A ratiometric sensor for DNA based on a dual emission Ru(dppz) light-switch complex. Dalton Trans. 2017, 46, 6079–6086. [Google Scholar] [CrossRef]
- Hudson, Z.M.; Zhao, S.B.; Wang, R.Y.; Wang, S. Switchable Ambient-Temperature Singlet–Triplet Dual Emission in Non-conjugated Donor–Acceptor Triarylboron–PtII Complexes. Chem. Eur. J. 2009, 15, 6131–6137. [Google Scholar] [CrossRef]
- Kwak, S.W.; Choi, B.H.; Lee, J.H.; Hwang, H.; Lee, J.; Kwon, H.; Chung, Y.; Lee, K.M.; Park, M.H. Synthesis and Dual-Emission Feature of Salen-Al/Triarylborane Dyads. Inorg. Chem. 2017, 56, 6039–6043. [Google Scholar] [CrossRef]
- Kumar, S.; Hisamatsu, Y.; Tamaki, Y.; Ishitani, O.; Aoki, S. Design and Synthesis of Heteroleptic Cyclometalated Iridium(III) Complexes Containing Quinoline-Type Ligands that Exhibit Dual Phosphorescence. Inorg. Chem. 2016, 55, 3829–3843. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Liu, H.-W.; Tang, M.-C.; Choi, A.W.-T.; Zhu, N.; Wei, X.-G.; Lau, K.-C.; Lo, K.K.-W. Dual-Emissive Cyclometalated Iridium(III) Polypyridine Complexes as Ratiometric Biological Probes and Organelle-Selective Bioimaging Reagents. Inorg. Chem. 2015, 54, 6582–6593. [Google Scholar] [CrossRef]
- De Cola, L.; Barigelletti, F.; Balzani, V.; Belser, P.; Von Zelewsky, A.; Seel, C.; Frank, M.; Vögtle, F. Polynuclear complexes of tris(bipyridine) bridging ligands. Energy transfer from Ru-based to Os-based components. Coord. Chem. Rev. 1991, 111, 255–260. [Google Scholar] [CrossRef]
- Aguirre-Etcheverry, P.; O’Hare, D. Electronic Communication through Unsaturated Hydrocarbon Bridges in Homobimetallic Organometallic Complexes. Chem. Rev. 2010, 110, 4839–4864. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, D.; Wang, X.; Su, Z.; Bryce, M.R. Dinuclear metal complexes: Multifunctional properties and applications. Chem. Soc. Rev. 2020, 49, 765–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazer, E.C.; Magde, D.; Tor, Y. Dual Emission from a Family of Conjugated Dinuclear RuII Complexes. J. Am. Chem. Soc. 2005, 127, 4190–4192. [Google Scholar] [CrossRef]
- Han, M.; Tian, Y.; Yuan, Z.; Zhu, L.; Ma, B. A Phosphorescent Molecular “Butterfly” that undergoes a Photoinduced Structural Change allowing Temperature Sensing and White Emission. Angew. Chem. Int. Ed. 2014, 53, 10908–10912. [Google Scholar] [CrossRef]
- Zhou, C.; Tian, Y.; Yuan, Z.; Han, M.; Wang, J.; Zhu, L.; Tameh, M.S.; Huang, C.; Ma, B. Precise Design of Phosphorescent Molecular Butterflies with Tunable Photoinduced Structural Change and Dual Emission. Angew. Chem. Int. Ed. 2015, 54, 9591–9595. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Shao, J.-Y.; Gong, Z.-L.; Chen, N.; Zhong, Y.-W. Tuning the dual emissions of a monoruthenium complex with a dangling coordination site by solvents, O2, and metal ions. Dalton Trans. 2017, 47, 292–297. [Google Scholar] [CrossRef]
- Cárdenas, D.J.; Echavarren, A.M.; De Arellano, M.C.R. Divergent Behavior of Palladium(II) and Platinum(II) in the Metalation of 1,3-Di(2-pyridyl)benzene. Organometallics 1999, 18, 3337–3341. [Google Scholar] [CrossRef]
- Cave, G.W.V.; Alcock, A.N.W.; Rourke, J.P. High-Yield Synthesis of a C∧N∧C Tridentate Platinum Complex. Organometallics 1999, 18, 1801–1803. [Google Scholar] [CrossRef]
- Constable, E.C.; Henney, P.G.; Leese, T.A.; Tocher, D.A. Cyclometallation Reactions of 6-Phenyl-2,2’-bipyridine; a Potential C,N,N-Donor Analogue of 2,2’:6’,2"-Terpyridine. Crystal and Molecular Structure of Dichloro-bis(6-phenyl-2,2’-bipyridine)ruthenium(II). J. Chem. Soc. Dalton Trans. 1990, 2, 443–449. [Google Scholar] [CrossRef]
- Wu, S.H.; Burkhardt, S.E.; Yao, J.; Zhong, Y.W.; Abruña, H.D. Near-Infrared Absorbing and Emitting RuII-PtII Heterodimetallic Complexes of Dpdpz (Dpdpz = 2,3-Di(2-pyridyl)-5,6-diphenylpyrazine). Inorg. Chem. 2011, 50, 3959–3969. [Google Scholar] [CrossRef]
- Xue, F.; Lu, Y.; Zhou, Z.; Shi, M.; Yan, Y.; Yang, H.; Yang, S. Two in One: Luminescence Imaging and 730 nm Continuous Wave Laser Driven Photodynamic Therapy of Iridium Complexes. Organometallics 2014, 34, 73–77. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, R.; Li, Y.-J.; Li, Y.-Z.; Zuo, J.-L.; You, X.-Z. Syntheses, Structures, and Electrochemical Properties of Platinum(II) Complexes Containing Di-tert-butylbipyridine and Crown Ether Annelated Dithiolate Ligands. Inorg. Chem. 2007, 46, 866–873. [Google Scholar] [CrossRef]
- Hua, F.; Kinayyigit, S.; Rachford, A.A.; Shikhova, E.A.; Goeb, S.; Cable, J.R.; Adams, C.J.; Kirschbaum, K.; Pinkerton, A.A.; Castellano, F.N. Luminescent Charge-Transfer Platinum(II) Metallacycle. Inorg. Chem. 2007, 46, 8771–8783. [Google Scholar] [CrossRef] [Green Version]
- Ventura, B.; Barbieri, A.; Barigelletti, F.; Seneclauze, J.B.; Retailleau, P.; Ziessel, R. Trichromophoric Systems from Square-Planar Pt-Ethynylbipyridine and Octahedral Ru- and Os-Bipyridine Centers: Syntheses, Structures, Electrochemical Behavior, and Bipartition of Energy Transfer. Inorg. Chem. 2008, 47, 7048–7058. [Google Scholar] [CrossRef]
- Wong, K.M.C.; Yam, V.W.W. Self-Assembly of Luminescent Alkynylplatinum(II) Terpyridyl Complexes: Modulation of Pho-tophysical Properties through Aggregation Behavior. Acc. Chem. Res. 2011, 44, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Aliprandi, A.; Genovese, D.; Mauro, M.; De Cola, L. Recent Advances in Phosphorescent Pt(II) Complexes Featuring Metal-lophilic Interactions: Properties and Applications. Chem. Lett. 2015, 44, 1152–1169. [Google Scholar] [CrossRef]
- Ionkin, A.S.; Marshall, W.J.; Wang, Y. Syntheses, Structural Characterization, and First Electroluminescent Properties of Mono-cyclometalated Platinum(II) Complexes with Greater than Classical π-π Stacking and Pt-Pt Distances. Organometallics 2005, 24, 619–627. [Google Scholar] [CrossRef]
- Tinker, L.L.; Bernhard, S. Photon-Driven Catalytic Proton Reduction with a Robust Homoleptic Iridium(III) 6-Phenyl-2,2′-bipyridine Complex ([Ir(C/\N/\N)2]+). Inorg. Chem. 2009, 48, 10507–10511. [Google Scholar] [CrossRef]
- Cho, Y.J.; Kim, S.Y.; Choi, C.M.; Kim, N.J.; Kim, C.H.; Cho, D.W.; Son, H.J.; Pac, C.; Kang, S.O. Photophysics and Excited-State Properties of Cyclometalated Iridium(III)–Platinum(II) and Iridium(III)–Iridium(III) Bimetallic Complexes Bridged by Dipyri-dylpyrazine. Inorg. Chem. 2017, 56, 5305–5315. [Google Scholar] [CrossRef]
- Tamayo, A.B.; Garon, S.; Sajoto, T.; Djurovich, P.I.; Tsyba, I.M.; Bau, R.; Thompson, M.E. Cationic Bis-cyclometalated Iridi-um(III) Diimine Complexes and Their Use in Efficient Blue, Green, and Red Electroluminescent Devices. Inorg. Chem. 2005, 44, 8723–8732. [Google Scholar] [CrossRef] [PubMed]
- Kisel, K.S.; Melnikov, A.S.; Grachova, E.V.; Hirva, P.; Tunik, S.P.; Koshevoy, I.O. Linking ReI and PtII Chromophores with Aminopyridines: A Simple Route to Achieve a Complicated Photophysical Behavior. Chem. A Eur. J. 2017, 23, 11301–11311. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.M.-C.; Hui, C.-K.; Yu, K.-L.; Yam, V.W.-W. Luminescence studies of dinuclear platinum(II) alkynyl complexes and their mixed-metal platinum(II)–copper(I) and –silver(I) complexes. Coord. Chem. Rev. 2002, 229, 123–132. [Google Scholar] [CrossRef]
- Panigati, M.; Mauro, M.; Donghi, D.; Mercandelli, P.; Mussini, P.; De Cola, L.; D’Alfonso, G. Luminescent dinuclear rhenium(I) complexes containing bridging 1,2-diazine ligands: Photophysical properties and application. Coord. Chem. Rev. 2012, 256, 1621–1643. [Google Scholar] [CrossRef]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]






| Compound | λabs(max)/nm (ε/105 M−1cm−1) | λem(max)b/nm | τ (N2) c/ns | Φd |
|---|---|---|---|---|
| 1 | 309 (0.13), 369 (0.07) | 460 | 10 | 43% |
| 2 | 284 (0.24), 305 (0.22), 402 (0.07) | 432 | 6 (at 410 nm) | 3.2% |
| 3 | 252 (1.13), 303 (0.62), 370 (0.15) | 454 | 135 (at 480 nm) | 5.8% |
| 4 | 264 (0.96), 317 (0.43), 377 (0.21), 491 (0.02) | 420/520 | 8/99 | 1.5% |
| Compound | Sn | E (ev) | λ (nm) | f | Dominant Transition(s) (Percentage Contribution b) | Assignment c |
|---|---|---|---|---|---|---|
| 2 | 1 | 3.10 | 399 | 0.0648 | HOMO → LUMO (77%) | ILdapzCT |
| 2 | 3.15 | 394 | 0.0245 | HOMO–1 → LUMO + 1 (34%) HOMO–2 → LUMO + 1 (28%) | MC | |
| 7 | 3.61 | 343 | 0.033 | HOMO → LUMO + 2 (50%) | ILdapzCT | |
| 8 | 3.68 | 337 | 0.0217 | HOMO → LUMO + 2 (35%) | ILdapzCT | |
| 10 | 3.90 | 318 | 0.122 | HOMO–3 → LUMO (70%) | MPtLdapzCT | |
| 3 | 1 | 2.99 | 415 | 0.0244 | HOMO → LUMO (97%) | MIrLdapzCT |
| 2 | 3.14 | 395 | 0.052 | HOMO → LUMO + 1 (89%) | MIrLppyCT/LdapzLppyCT | |
| 4 | 3.26 | 381 | 0.0565 | HOMO–1 → LUMO (87%) | MIrLdapzCT/LppyLdapzCT | |
| 5 | 3.52 | 353 | 0.0369 | HOMO → LUMO + 3 (85%) | MIrLdapzCT/LppyLdapzCT | |
| 11 | 3.83 | 324 | 0.1018 | HOMO–1 → LUMO + 3 (84%) | MIrLdapzCT/LppyLdapzCT | |
| 4 | 1 | 2.25 | 551 | 0.011 | HOMO → LUMO (99%) | MIrLdapzCT/LppyLdapzCT |
| 2 | 2.88 | 430 | 0.057 | HOMO–2 → LUMO (58%) HOMO–1 → LUMO (32%) | MIrLdapzCT/ILdapzCT | |
| 4 | 3.04 | 408 | 0.0301 | HOMO–3 → LUMO (61%) | MIrLdapzCT/LppyLdapzCT | |
| 9 | 3.20 | 388 | 0.062 | HOMO → LUMO+2 (91%) | MC | |
| 12 | 3.30 | 375 | 0.0408 | HOMO–5 → LUMO (17%) HOMO–5 → LUMO + 1 (19%) | MIrLdapzCT/MPtLdapzCT | |
| 14 | 3.40 | 364 | 0.0322 | HOMO–9 → LUMO (50%) | MPtLdapzCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-H.; Ma, D.-X.; Gong, Z.-L.; Ma, J.; Shao, J.-Y.; Yang, R.; Zhong, Y.-W. Synthesis, Photophysical, and Computational Studies of a Bridged IrΙΙΙ-PtΙΙ Heterodimetallic Complex. Crystals 2021, 11, 236. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11030236
Wu S-H, Ma D-X, Gong Z-L, Ma J, Shao J-Y, Yang R, Zhong Y-W. Synthesis, Photophysical, and Computational Studies of a Bridged IrΙΙΙ-PtΙΙ Heterodimetallic Complex. Crystals. 2021; 11(3):236. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11030236
Chicago/Turabian StyleWu, Si-Hai, Dian-Xue Ma, Zhong-Liang Gong, Junjie Ma, Jiang-Yang Shao, Rong Yang, and Yu-Wu Zhong. 2021. "Synthesis, Photophysical, and Computational Studies of a Bridged IrΙΙΙ-PtΙΙ Heterodimetallic Complex" Crystals 11, no. 3: 236. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11030236