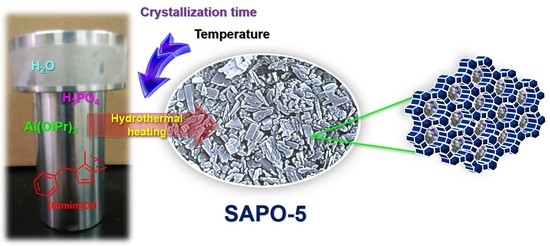
Effects of Synthesis Parameters on the Crystallization Profile and Morphological Properties of SAPO-5 Templated by 1-Benzyl-2,3-Dimethylimidazolium Hydroxide
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of 1-Benzyl-2,3-Dimethylimidazolium Chloride, [bzmIm]Cl
2.2. Preparation of 1-Benzyl-2,3-Dimethylimidazolium Hydroxide ([bzmIm]OH) Solution

2.3. Synthesis of SAPO-5 Microporous Solids
2.4. Characterization
3. Results and Discussion
3.1. Effect of Crystallization Time
3.2. Effect of P2O5/Al2O3 Molar Ratio
3.3. Effect of [bzmIm]2O/P2O5 Molar Ratio
3.4. Effect of H2O Content
3.5. Effect of SiO2/Al2O3 Molar Ratio
3.6. Effect of Heating Temperature
3.7. Formation Pathway of SAPO-5 Crystals
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tosheva, L.; Ng, E.-P.; Mintova, S.; Hölzl, M.; Metzger, T.H.; Doyle, A.M. AlPO4-18 seed layers and films by secondary growth. Chem. Mater. 2008, 20, 5721–5726. [Google Scholar] [CrossRef]
- Ng, E.-P.; Awala, H.; Komaty, S.; Mintova, S. Microwave-green synthesis of AlPO-n and SAPO-n (n= 5 and 18) nanosized crystals and their assembly in layers. Microporous Mesoporous Mater. 2019, 280, 256–263. [Google Scholar] [CrossRef]
- IZA-SC Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 26 January 2021).
- Li, M.; Zeng, C.; Zhang, L. Hydrothermal synthesis of SAPO-5 with novel morphologies from hydrogels containing acetic acid and high concentration of triethylamine under neutral or alkaline conditions. CrystEngComm 2012, 14, 3787–3792. [Google Scholar] [CrossRef]
- Gómez-Hortigüela, L.; Pérez-Pariente, J.; Corà, F.; Catlow, C.R.A.; Blasco, T. Structure-directing role of molecules containing benzyl rings in the synthesis of a large-pore aluminophosphate molecular sieve: An experimental and computational study. J. Phys. Chem. B 2005, 109, 21539–21548. [Google Scholar] [CrossRef]
- Jiang, F.; Tang, Z.; Zhai, J.; Ye, J.; Han, J. Synthesis of AlPO4-5 crystals using TBAOH as template. Microporous Mesoporous Mater. 2006, 92, 129–133. [Google Scholar] [CrossRef]
- Ng, E.-P.; Delmotte, L.; Mintova, S. Environmentally benign synthesis of nanosized aluminophosphate enhanced by microwave heating. Green Chem. 2008, 10, 1043–1048. [Google Scholar] [CrossRef]
- Roldán, R.; Sánchez-Sánchez, M.; Sankar, G.; Romero-Salguero, F.J.; Jiménez-Sanchidrián, C. Influence of pH and Si content on Si incorporation in SAPO-5 and their catalytic activity for isomerisation of n-heptane over Pt loaded catalysts. Microporous Mesoporous Mater. 2007, 99, 288–298. [Google Scholar] [CrossRef]
- Geng, L.; Dong, H.; Liu, X.; Zhang, B. Efficient manipulation of continuous AFI-type aluminophosphate membranes with distinctive microstructures on macroporous α-Al2O3 substrates. Molecules 2018, 23, 1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auwal, I.A.; Mintova, S.; Ling, T.C.; Khoerunnisa, F.; Wong, K.-L.; Ng, E.-P. Crystallization profile and morphological study of SAPO-5 templated by imidazolium cations of different functional groups. Microporous Mesoporous Mater. 2020, 308, 110514. [Google Scholar] [CrossRef]
- Khoo, D.Y.; Kok, W.-M.; Mukti, R.R.; Mintova, S.; Ng, E.-P. Ionothermal approach for synthesizing AlPO-5 with hexagonal thin-plate morphology influenced by various parameters at ambient pressure. Solid State Sci. 2013, 25, 63–69. [Google Scholar] [CrossRef]
- Ng, E.-P.; Sekhon, S.S.; Mintova, S. Discrete MnAlPO-5 nanocrystals synthesized by an ionothermal approach. Chem. Commun. 2009, 13, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.P.; Itani, L.; Sekhon, S.S.; Mintova, S. Micro-to Macroscopic Observations of MnAlPO-5 Nanocrystal Growth in Ionic-Liquid Media. Chem. Eur. J. 2010, 16, 12890–12897. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.-P.; Wong, K.-L.; Ng, D.T.-L.; Awala, H.; Mukti, R.R.; Adam, F.; Mintova, S. AlPO-5 nanocrystals templated by 1-ethyl-2, 3-dimethylimidazolium hydroxide and their textural and water sorption properties. Mater. Chem. Phys. 2017, 188, 49–57. [Google Scholar] [CrossRef]
- Khoo, D.Y.; Awala, H.; Mintova, S.; Ng, E.-P. Synthesis of AlPO-5 with diol-substituted imidazolium-based organic template. Microporous Mesoporous Mater. 2014, 194, 200–207. [Google Scholar] [CrossRef]
- Ghrear, T.M.A.; Rigolet, S.; Daou, T.J.; Mintova, S.; Ling, T.C.; Tan, S.H.; Ng, E.-P. Synthesis of Cs-ABW nanozeolite in organotemplate-free system. Microporous Mesoporous Mater. 2019, 277, 78–83. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, S.; Li, P.; Li, X.; Xu, Y.; Dong, Y.; Song, W.; Yi, X.; Fang, W. Fabricating self-assembled SAPO-5 with tailored mesoporosity and acidity using a single template. CrystEngComm 2017, 19, 5275–5284. [Google Scholar] [CrossRef]
- Auwal, I.A.; Wong, K.-L.; Ling, T.C.; Ooi, B.S.; Ng, E.-P. Metal Chlorides Grafted on SAPO-5 (MClx/SAPO-5) as Reusable and Superior Catalysts for Acylation of 2-Methylfuran Under Non-Microwave Instant Heating Condition. Processes 2020, 8, 603. [Google Scholar] [CrossRef]
- Agliullin, M.; Kutepov, B. Selective Crystallization of AlPO 4-41 Molecular Sieve in the Presence of Diethylamine. Petrol. Chem. 2020, 60, 890–894. [Google Scholar] [CrossRef]
- Ng, E.-P.; Awala, H.; Ghoy, J.-P.; Vicente, A.; Ling, T.C.; Ng, Y.H.; Mintova, S.; Adam, F. Effects of ultrasonic irradiation on crystallization and structural properties of EMT-type zeolite nanocrystals. Mater. Chem. Phys. 2015, 159, 38–45. [Google Scholar] [CrossRef]
- Wragg, D.S.; Slawin, A.M.; Morris, R.E. The role of added water in the ionothermal synthesis of microporous aluminium phosphates. Solid State Sci. 2009, 11, 411–416. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, C.; Zhang, Y.; Ren, N.; Tang, Y. Microwave-assisted hydrothermal synthesis of nanozeolites with controllable size. Microporous Mesoporous Mater. 2009, 119, 306–314. [Google Scholar] [CrossRef]
- Wong, S.-F.; Deekomwong, K.; Wittayakun, J.; Ling, T.C.; Muraza, O.; Adam, F.; Ng, E.-P. Crystal growth study of KF nanozeolite and its catalytic behavior in Aldol condensation of benzaldehyde and heptanal enhanced by microwave heating. Mater. Chem. Phys. 2017, 196, 295–301. [Google Scholar] [CrossRef]
- Askari, S.; Siahmard, A.B.; Halladj, R.; Alipour, S.M. Different techniques and their effective parameters in nano SAPO-34 synthesis: A review. Powder Technol. 2016, 301, 268–287. [Google Scholar] [CrossRef]
- Chen, J.; Wright, P.; Natarajan, S.; Thomas, J. Understanding the Brønsted acidity of SAPO-5, SAPO-17, SAPO-18 and SAPO-34 and their catalytic performance for methanol conversion to hydrocarbons. Stud. Surf. Sci. Catal. 1994, 84, 1731–1738. [Google Scholar]
- Persson, A.; Schoeman, B.; Sterte, J.; Otterstedt, J.-E. The synthesis of discrete colloidal particles of TPA-silicalite-1. Zeolites 1994, 14, 557–567. [Google Scholar] [CrossRef]
- Brar, T.; France, P.; Smirniotis, P.G. Control of crystal size and distribution of zeolite A. Ind. Eng. Chem. Res. 2001, 4, 1133–1139. [Google Scholar] [CrossRef]
- An, T.; An, J.; Yang, H.; Li, G.; Feng, H.; Nie, X. Photocatalytic degradation kinetics and mechanism of antivirus drug-lamivudine in TiO2 dispersion. J. Hazard. Mater. 2011, 197, 229–236. [Google Scholar] [CrossRef]
- Garofalini, S.H.; Miller, A. Kinetics of tridymite formation. J. Cryst. Growth 1986, 78, 85–96. [Google Scholar] [CrossRef]
- Wang, R.; Lin, C.; Ho, Y.; Leu, L.; Chao, K. Silicon species in a SAPO-5 molecular sieve. Appl. Catal. 1991, 72, 39–49. [Google Scholar] [CrossRef]
- Erichsen, M.W.; Svelle, S.; Olsbye, U. H-SAPO-5 as methanol-to-olefins (MTO) model catalyst: Towards elucidating the effects of acid strength. Catalysis 2013, 298, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Pang, W.; Yu, J.; Huo, Q.; Chen, J. Chemistry of Zeolites and Related Porous Materials: Synthesis and Structure; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Briend, M.; Peltre, M.; Massiani, P.; Man, P.; Vomscheid, R.; Derewinski, M.; Barthomeuf, D. Modifications of structure and Si environment upon heating of SAPO-5, SAPO-34 and SAPO-37. Stud. Surf. Sci. Catal. 1994, 98, 613–620. [Google Scholar]
- Grand, J.; Awala, H.; Mintova, S. Mechanism of zeolites crystal growth: New findings and open questions. CrystEngComm 2016, 18, 650–664. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef]
- Wong, S.-F.; Awala, H.; Vincente, A.; Retoux, R.; Ling, T.C.; Mintova, S.; Mukti, R.R.; Ng, E.-P. KF zeolite nanocrystals synthesized from organic-template-free precursor mixture. Microporous Mesoporous Mater. 2017, 249, 105–110. [Google Scholar] [CrossRef]
- Jorge, M.; Auerbach, S.M.; Monson, P.A. Modeling spontaneous formation of precursor nanoparticles in clear-solution zeolite synthesis. J. Am. Chem. Soc. 2005, 127, 14388–14400. [Google Scholar] [CrossRef] [Green Version]
- Auerbach, S.M.; Ford, M.H.; Monson, P. New insights into zeolite formation from molecular modeling. Curr. Opin. Colloid Interface Sci. 2005, 10, 220–225. [Google Scholar] [CrossRef]
- Kumar, A.; Molinero, V. Two-step to one-step nucleation of a zeolite through a metastable gyroid mesophase. J. Phys. Chem. Lett. 2018, 9, 5692–5697. [Google Scholar] [CrossRef] [PubMed]
- Jhung, S.H.; Chang, J.-S.; Kim, D.S.; Park, S.-E. Effects of silica on the synthesis of AFI molecular sieve in acid and base conditions under microwave irradiation. Microporous Mesoporous Mater. 2004, 71, 135–142. [Google Scholar] [CrossRef]
- Chen, C.-M.; Jehng, J.-M. Effect of synthesis pH and H 2 O molar ratio on the structure and morphology of aluminum phosphate (AlPO-5) molecular sieves. Catal. Lett. 2003, 85, 73–80. [Google Scholar] [CrossRef]
- Ng, E.-P.; Mohammad, S.A.G.; Rigolet, S.; Daou, T.J.; Mintova, S.; Ling, T.C. Micro-and macroscopic observations of the nucleation process and crystal growth of nanosized Cs-pollucite in an organotemplate-free hydrosol. New J. Chem. 2019, 43, 17433–17440. [Google Scholar] [CrossRef]













| Synthesis Parameters | Samples | Gel Molar Composition | T (°C) | t (h) | Si/(P + Al + Si) Ratio * | Products † | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | P2O5 | [bzmIm]2O | SiO2 | H2O | ||||||
| Time | SH1 | 1 | 2.5 | 2.5 | 0.47 | 180 | 150 | 2 | 0.008 | Amor. |
| SH2 | 4 | 0.021 | Amor. | |||||||
| SH3 | 8 | 0.051 | Amor. + AFI | |||||||
| SH4 | 10 | 0.056 | AFI | |||||||
| SH5 | 14 | 0.057 | AFI + AFO | |||||||
| P2O5 | SH6 | 1 | 2.0 | 2.5 | 0.47 | 180 | 150 | 10 | - | No solid |
| SH4 | 2.5 | 0.056 | AFI | |||||||
| SH7 | 3.0 | 0.062 | AFI | |||||||
| SH8 | 3.5 | 0.067 | AFI + cristobalite | |||||||
| P2O5 /[bzmIm]2O | SH9 | 1 | 1.5 | 1.5 | 0.47 | 180 | 150 | 10 | 0.059 | AFI |
| SH10 | 2.0 | 2.0 | 0.057 | AFI | ||||||
| SH4 | 2.5 | 2.5 | 0.056 | AFI | ||||||
| SH11 | 3.0 | 3.0 | 0.053 | AFI | ||||||
| H2O | SH12 | 1 | 2.5 | 2.5 | 0.47 | 135 | 150 | 10 | 0.053 | Amor. + AFI |
| SH4 | 180 | 0.056 | AFI | |||||||
| SH13 | 225 | 0.057 | AFI | |||||||
| SH14 | 270 | 0.061 | Tridymite | |||||||
| SiO2 | SH15 | 1 | 2.5 | 2.5 | 0 | 180 | 150 | 10 | 0 | AFI (AlPO-5) |
| SH16 | 0.23 | 0.031 | AFI | |||||||
| SH4 | 0.47 | 0.056 | AFI | |||||||
| SH17 | 0.7 | 0.061 | AFI | |||||||
| Temperature | SH18 | 1 | 2.5 | 2.5 | 0.47 | 180 | 100 | 10 | 0.010 | Amor. |
| SH19 | 120 | 0.021 | AFI | |||||||
| SH4 | 150 | 0.056 | AFI | |||||||
| SH20 | 200 | 0.064 | Tridymite | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auwal, I.A.; Khoerunnisa, F.; Dubray, F.; Mintova, S.; Ling, T.C.; Wong, K.-L.; Ng, E.-P. Effects of Synthesis Parameters on the Crystallization Profile and Morphological Properties of SAPO-5 Templated by 1-Benzyl-2,3-Dimethylimidazolium Hydroxide. Crystals 2021, 11, 279. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11030279
Auwal IA, Khoerunnisa F, Dubray F, Mintova S, Ling TC, Wong K-L, Ng E-P. Effects of Synthesis Parameters on the Crystallization Profile and Morphological Properties of SAPO-5 Templated by 1-Benzyl-2,3-Dimethylimidazolium Hydroxide. Crystals. 2021; 11(3):279. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11030279
Chicago/Turabian StyleAuwal, Ismail Alhassan, Fitri Khoerunnisa, Florent Dubray, Svetlana Mintova, Tau Chuan Ling, Ka-Lun Wong, and Eng-Poh Ng. 2021. "Effects of Synthesis Parameters on the Crystallization Profile and Morphological Properties of SAPO-5 Templated by 1-Benzyl-2,3-Dimethylimidazolium Hydroxide" Crystals 11, no. 3: 279. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11030279