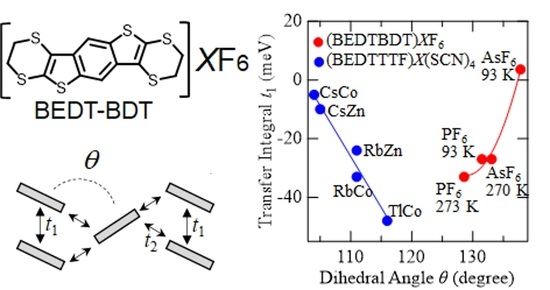
Dihedral-Angle Dependence of Intermolecular Transfer Integrals in BEDT-BDT-Based Radical-Cation Salts with θ-Type Molecular Arrangements
Abstract
:1. Introduction
2. Experimental Methods
2.1. Electrocrystallization
2.2. X-ray Diffraction and Structural Analysis
2.3. Calculation of Transfer Integrals and Fermi Surfaces
2.4. Resistivity Measurements
2.5. Magnetic-Susceptibility Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ishiguro, T.; Yamaji, K.; Saito, G. Organic Superconductors, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Mori, T. Electronic Properties of Organic Conductors; Springer: Tokyo, Japan, 2016. [Google Scholar]
- Mori, T. Principles that Govern Electronic Transport in Organic Conductors and Transistors. Bull. Chem. Soc. Jpn. 2016, 89, 973–986. [Google Scholar] [CrossRef] [Green Version]
- Morawitz, H. Orientational Peierls Transition in Quasi One-Dimensional Organic Solids. Phys. Rev. Lett. 1975, 34, 1096–1099. [Google Scholar] [CrossRef]
- Mori, H. Materials Viewpoint of Organic Superconductors. J. Phys. Soc. Jpn. 2006, 75, 051003. [Google Scholar] [CrossRef]
- Saito, G.; Yoshida, Y. Development of Conductive Organic Molecular Assemblies: Organic Metals, Superconductors, and Exotic Functional Materials. Bull. Chem. Soc. Jpn. 2007, 80, 1–137. [Google Scholar] [CrossRef]
- Shirahata, T.; Kohno, S.; Furuta, K.; Oka, Y.; Misaki, Y. Synthesis of New Electron Donor ClMe3-TTP: Structures and Properties of (ClMe3-TTP)3X (X = PF6 and AsF6). Bull. Chem. Soc. Jpn. 2015, 88, 1086–1092. [Google Scholar] [CrossRef]
- Kadoya, T.; Ashizawa, M.; Higashino, T.; Kawamoto, T.; Kumeta, S.; Matsumoto, H.; Mori, T. A Highly Conducting Organic Metal Derived from an Organic-Transistor Material: Benzothienobenzothiophene. Phys. Chem. Chem. Phys. 2013, 15, 17818–17822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyota, Y.; Kadoya, T.; Yamamoto, K.; Iijima, K.; Higashino, T.; Kawamoto, T.; Takimiya, K.; Mori, T. Benzothienobenzothiophene-Based Molecular Conductors: High Conductivity, Large Thermoelectric Power Factor, and One-Dimensional Instability. J. Am. Chem. Soc. 2016, 138, 3920–3925. [Google Scholar] [CrossRef]
- Higashino, T.; Kadoya, T.; Kumeta, S.; Kurata, K.; Kawamoto, T.; Mori, T. An Organic Metal Derived from a Selenium Analogue of Benzothienobenzothiophene. Eur. J. Inorg. Chem. 2014, 24, 3895–3898. [Google Scholar] [CrossRef]
- Kadoya, T.; Oki, R.; Kiyota, Y.; Koyama, Y.; Higashino, T.; Kubo, K.; Mori, T.; Yamada, J. Transport Properties of Molecular Conductors (BSBS)2XF6 (X = As, Sb, and Ta): Investigation of Intermolecular Transfer Integrals in the Radical-Cationic State of BTBT-Type Semiconductors. J. Phys. Chem. C 2019, 123, 5216–5222. [Google Scholar] [CrossRef]
- Kadoya, T.; Sugiura, S.; Tahara, K.; Higashino, T.; Kubo, K.; Sasaki, T.; Takimiya, K.; Yamada, J. Two-Dimensional Radical-Cationic Mott Insulator Based on an Electron Donor Containing Neither Tetrathiafulvalene nor Tetrathiapentalene Skeleton. CrystEngComm 2020, 22, 5949–5953. [Google Scholar] [CrossRef]
- Wang, C.; Nakamura, H.; Sugino, H.; Takimiya, K. Thiacycle-fused benzo[1,2-b:4,5-b′]dithiophenes (BDTs): Synthesis, Packing, Molecular Orientation and Semiconducting Properties. J. Mater. Chem. C 2018, 6, 3604–3612. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal Structure Determination and Refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta. Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ADF: Powerful DFT Code for Modeling Molecules; Scientific Computing and Modeling: Amsterdam, The Netherlands. Available online: http://www.scm.com/ADF/ (accessed on 30 June 2021).
- Mori, T.; Kobayashi, A.; Sasaki, Y.; Kobayashi, H.; Saito, G.; Inokuchi, H. The Intermolecular Interaction of Tetrathiafulvalene and Bis(ethylenedithio)tetrathiafulvalene in Organic Metals. Calculation of Orbital Overlaps and Models of Energy-band Structures. Bull. Chem. Soc. Jpn. 1984, 57, 627–633. [Google Scholar] [CrossRef]
- Ami, T.; Crawford, M.K.; Harlow, R.L.; Wang, Z.R.; Johnston, D.C.; Huang, Q.; Erwin, R.W. Magnetic Susceptibility and Low-Temperature Structure of the Linear Chain Cuprate Sr2CuO3. Phys. Rev. B 1995, 51, 5994. [Google Scholar] [CrossRef]
- Kojima, H.; Mori, T. Dihedral Angle Dependence of Transfer Integrals in Organic Semiconductors with Herringbone Structures. Bull. Chem. Soc. Jpn. 2011, 84, 1049–1056. [Google Scholar] [CrossRef]
- Mori, H.; Tanaka, S.; Mori, T.; Kobayashi, A.; Kobayashi, H. Crystal Structure and Physical Properties of M = Rb and Tl Salts (BEDT-TTF)2MM′(SCN)4 [M′ = Co, Zn]. Bull. Chem. Soc. Jpn. 1998, 71, 797–806. [Google Scholar] [CrossRef]
- Mori, H.; Tanaka, S.; Mori, T. Systematic study of the electronic state in θ-type BEDT-TTF organic conductors by changing the electronic correlation. Phys. Rev. B 1998, 57, 12023. [Google Scholar] [CrossRef]
- Mori, T. Structural Genealogy of BEDT-TTF-Based Organic Conductors I. Parallel Molecules: β and β′′ Phases. Bull. Chem. Soc. Jpn. 1998, 71, 2509–2526. [Google Scholar] [CrossRef]
- Mori, T.; Mori, H.; Tanaka, S. Structural Genealogy of BEDT-TTF-Based Organic Conductors II. Inclined Molecules: θ, α, and κ Phases. Bull. Chem. Soc. Jpn. 1999, 72, 179–197. [Google Scholar] [CrossRef]
- Kawasugi, Y.; Yamamoto, H.M.; Hosoda, M.; Tajima, N.; Fukunaga, T.; Tsukagoshi, K.; Kato, R. Strain-Induced Superconductor/Insulator Transition and Field Effect in a Thin Single Crystal of Molecular Conductor. Appl. Phys. Lett. 2008, 92, 243508. [Google Scholar] [CrossRef]
- Yamamoto, H.M.; Nakano, M.; Suda, M.; Iwasa, Y.; Kawasaki, M.; Kato, R. A Strained Organic Field-Effect Transistor with a Gate-Tunable Superconducting Channel. Nat. Commun. 2013, 4, 2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, M.; Kawasugi, Y.; Minari, T.; Tsukagoshi, K.; Kato, R.; Yamamoto, H.M. Strain-Tunable Superconducting Field-Effect Transistor with an Organic Strongly-Correlated Electron System. Adv. Mater. 2014, 26, 3490–3495. [Google Scholar] [CrossRef] [PubMed]







| T (K) | HOMO (eV) | t1 (meV) | t2 (meV) | d (Å) | θ (°) | |
|---|---|---|---|---|---|---|
| (BEDT-BDT)PF6 | 273 | −4.50 | −33 | 22 | 3.947 | 128.6 |
| - | 93 | −4.56 | −27 | 23 | 3.901 | 131.5 |
| (BEDT-BDT)AsF6 | 270 | −4.58 | −27 | 22 | 3.955 | 133.1 |
| - | 93 | −4.52 | 3.6 | 20 | 3.910 | 137.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadoya, T.; Sugiura, S.; Higashino, T.; Tahara, K.; Kubo, K.; Sasaki, T.; Takimiya, K.; Yamada, J.-i. Dihedral-Angle Dependence of Intermolecular Transfer Integrals in BEDT-BDT-Based Radical-Cation Salts with θ-Type Molecular Arrangements. Crystals 2021, 11, 868. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11080868
Kadoya T, Sugiura S, Higashino T, Tahara K, Kubo K, Sasaki T, Takimiya K, Yamada J-i. Dihedral-Angle Dependence of Intermolecular Transfer Integrals in BEDT-BDT-Based Radical-Cation Salts with θ-Type Molecular Arrangements. Crystals. 2021; 11(8):868. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11080868
Chicago/Turabian StyleKadoya, Tomofumi, Shiori Sugiura, Toshiki Higashino, Keishiro Tahara, Kazuya Kubo, Takahiko Sasaki, Kazuo Takimiya, and Jun-ichi Yamada. 2021. "Dihedral-Angle Dependence of Intermolecular Transfer Integrals in BEDT-BDT-Based Radical-Cation Salts with θ-Type Molecular Arrangements" Crystals 11, no. 8: 868. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11080868