Growth of Acetaminophen Polymorphic Crystals and Solution-Mediated Phase Transition from Trihydrate to Form II in Agarose Gel
Abstract
:1. Introduction
2. Experiments
2.1. Sample Preparation
2.2. Diffusion of Coomassie Brilliant Blue in Agarose Gels
2.3. Acetaminophen Polymorphic Crystals in Agarose Gels
2.4. Solubility of Acetaminophen Polymorphic Crystals in Agarose Gels
2.5. Solution-Mediated Phase Transition from Acetaminophen Trihydrate to Form II in Agarose Gels
3. Results and Discussion
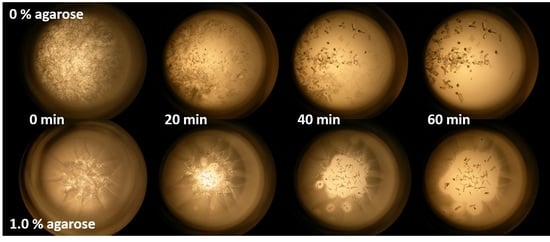
3.1. Diffusion of Coomassie Brilliant Blue in Agarose Gels
3.2. Acetaminophen Polymorphic Crystals in Agarose Gels
3.3. Solubility of Acetaminophen Polymorphic Crystals in Agarose Gels
3.4. Solution-Mediated Phase Transition from Acetaminophen Trihydrate to Form II in Agarose Gels
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Haleblian, J.; McCrone, W. Pharmaceutical applications of polymorphism. J. Pharm. Sci. 1969, 58, 911–929. [Google Scholar] [CrossRef]
- Chung, H.; Diao, Y. Polymorphism as an emerging design strategy for high performance organic electronics. J. Mater. Chem. C 2016, 4, 3915–3933. [Google Scholar] [CrossRef]
- Bhattachar, S.N.; Deschenes, L.A.; Wesley, J.A. Solubility: It’s not just for physical chemists. Drug Discov. Today 2006, 11, 1012–1018. [Google Scholar] [CrossRef]
- Tsuri, Y.; Maruyama, M.; Yoshikawa, H.Y.; Okada, S.; Adachi, H.; Takano, K.; Tsukamoto, K.; Imanishi, M.; Yoshimura, M.; Mori, Y. Intergrowth of two aspirin polymorphism observed with Raman spectroscopy. J. Cryst. Growth 2020, 532, 125430. [Google Scholar] [CrossRef]
- Censi, R.; Martino, P.D. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, H.; Kumar, S.; Roy, S.; Gaud, R. Patent protection strategies. J. Pharm. Bioallied. Sci. 2010, 2, 2–7. [Google Scholar] [CrossRef]
- Thayer, A.M. Centering on chirality. Chem. Eng. News 2007, 85, 11–19. [Google Scholar]
- Sugiyama, S.; Maruyama, M.; Sazaki, G.; Hirose, M.; Adachi, H.; Takano, K.; Murakami, S.; Inoue, T.; Mori, Y.; Matsumura, H. Growth of protein crystals in hydrogels prevents osmotic shock. J. Am. Chem. Soc. 2012, 134, 5786–5789. [Google Scholar] [CrossRef]
- Sugiyama, S.; Shimizu, N.; Kakinouchi, K.; Hiraoka, O.; Matsumura, H.; Yoshikawa, H.Y.; Takahashi, Y.; Maruyama, M.; Yoshimura, M.; Adachi, H.; et al. Development of protein seed crystals reinforced with high-strength hydrogels. CrystEngComm 2015, 17, 8064–8071. [Google Scholar] [CrossRef]
- Yoshikawa, H.Y.; Murai, R.; Sugiyama, S.; Sazaki, G.; Kitatani, T.; Takahashi, Y.; Adachi, H.; Matsumura, H.; Murakami, S.; Inoue, T.; et al. Femtosecond laser-induced nucleation of protein in agarose gel. J. Cryst. Growth 2009, 311, 956–959. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kakinouchi, K.; Adachi, H.; Maruyama, M.; Sugiyama, S.; Nakabayashi, I.; Tsuchikura, H.; Kuwahara, A.; Sano, S.; Yoshikawa, H.Y.; et al. Growth of high-strength protein crystals with nanofibers. Appl. Phys. Express 2016, 9, 035503. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kakinouchi, K.; Adachi, H.; Maruyama, M.; Sugiyama, S.; Sano, S.; Yoshikawa, H.Y.; Takahashi, Y.; Yoshimura, M.; Matsumura, H.; et al. Protein crystallization with paper. Jpn. J. Appl. Phys. 2016, 55, 050302. [Google Scholar] [CrossRef]
- Foster, J.A.; Piepenbrock, M.-O.M.; Lloyd, G.O.; Clarke, N.; Howard, J.A.K.; Steed, J.W. Anion-switchable supramolecular gels for controlling pharmaceutical crystal growth. Nat. Chem. 2010, 2, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, L.; Yao, C.; Wang, Z.; Xie, G.; Tao, X. Crystallization of sulfathiazole in gel: Polymorph selectivity and cross-nucleation. Cryst. Growth Des. 2020, 20, 9–16. [Google Scholar] [CrossRef]
- Maruyama, M.; Hayashi, Y.; Yoshikawa, H.Y.; Okada, S.; Koizumi, H.; Tachibana, M.; Sugiyama, S.; Adachi, H.; Matsumura, H.; Inoue, T.; et al. A crystallization technique for obtaining large protein crystals with increased mechanical stability using agarose gel combined with a stirring technique. J. Cryst. Growth 2016, 452, 172–178. [Google Scholar] [CrossRef]
- Jeppson, J.O.; Laurell, C.B.; Franzen, B. Agarose gel electrophoresis. Clin. Chem. 1979, 25, 629–638. [Google Scholar] [CrossRef]
- Gustavsson, P.-E.; Son, P.-O. Monolithic polysaccharide materials. J. Chromatogr. Libr. 2003, 67, 121–141. [Google Scholar]
- Sugiyama, S.; Tanabe, K.; Hirose, M.; Kitatani, T.; Hasenaka, H.; Takahashi, Y.; Adachi, H.; Takano, K.; Murakami, S.; Mori, Y.; et al. Protein crystallization in agarose gel with high strength: Developing an automated system for protein crystallographic processes. Jpn. J. Appl. Phys. 2009, 48, 075502. [Google Scholar] [CrossRef]
- Pernodet, N.; Maaloum, M.; Tinland, B. Pore size of agarose gels by atomic force microscopy. Electrophoresis 1997, 18, 55–58. [Google Scholar] [CrossRef]
- Pluen, A.; Netti, P.A.; Jain, R.K.; Berk, D.A. Diffusion of macromolecules in agarose gels: Comparison of linear and globular configurations. Biophys. J. 1999, 77, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, M.; Yoshimura, M.; Takano, K.; Mori, Y. Development of polymorphic control technology for pharmaceutical compounds. In Kinetic Control in Synthetic and Self-Assembly; Numata, M., Yagai, S., Hamura, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–291. [Google Scholar]
- Haisa, M.; Kashino, S.; Kawai, R.; Maeda, H. The monoclinic form of p-hydroxyacetanilide. Acta Crystallogr. Sect. B 1976, 32, 1283–1285. [Google Scholar] [CrossRef] [Green Version]
- Haisa, M.; Kashino, S.; Maeda, H. The orthorhombic form of p-hydroxyacetanilide. Acta Crystallogr. Sect. B 1974, 30, 2510–2512. [Google Scholar] [CrossRef]
- Burger, A. Interpretation of polymorphism studies. Acta Pharm. Technol. 1982, 28, 1–20. [Google Scholar]
- Parkin, A.; Parsons, S.; Pulham, C.R. Paracetamol monohydrate at 150 K. Acta Crystallogr. Sect. E 2002, 58, o1345–o1347. [Google Scholar] [CrossRef]
- Fabbiani, F.P.A.; Allan, D.R.; David, W.I.F.; Moggach, S.A.; Parsons, S.; Pulham, C.R. High-pressure recrystallisation—A route to new polymorphs and solvates. CrystEngComm 2004, 6, 505–511. [Google Scholar] [CrossRef]
- McGregor, P.A.; Allan, D.R.; Parsons, S.; Pulham, C.R. Preparation and crystal structure of a trihydrate of paracetamol. J. Pharm. Sci. 2002, 91, 1308–1311. [Google Scholar] [CrossRef]
- Mori, Y.; Maruyama, M.; Takahashi, Y.; Ikeda, K.; Fukukita, S.; Yoshikawa, H.; Okada, S.; Adachi, H.; Sugiyama, S.; Takano, K.; et al. Selective crystallization of metastable phase of acetaminophen by ultrasonic irradiation. Appl. Phys. Express 2015, 8, 065501. [Google Scholar] [CrossRef]
- Mori, Y.; Maruyama, M.; Takahashi, Y.; Yoshikawa, H.; Okada, S.; Adachi, H.; Sugiyama, S.; Takano, K.; Murakami, S.; Matsumura, H.; et al. Metastable crystal growth of acetaminophen using solution-mediated phase transformation. Appl. Phys. Express 2017, 10, 015501. [Google Scholar] [CrossRef]
- Mori, Y.; Maruyama, M.; Takahashi, Y.; Yoshikawa, H.; Okada, S.; Adachi, H.; Sugiyama, S.; Takano, K.; Murakami, S.; Matsumura, H.; et al. Crystallization of acetaminophen form II by plastic-ball-assisted ultrasonic irradiation. Appl. Phys. Express 2017, 10, 025501. [Google Scholar] [CrossRef]
- Nii, K.; Maruyama, M.; Okada, S.; Adachi, H.; Takano, K.; Murakami, S.; Yoshikawa, H.; Matsumura, H.; Inoue, T.; Imanishi, M.; et al. Improvement of metastable crystal of acetaminophen by control of crystal growth rate. Appl. Phys. Express 2018, 11, 035501. [Google Scholar] [CrossRef]
- Fujimoto, R.; Maruyama, M.; Mori, Y.; Okada, S.; Adachi, H.; Yoshikawa, H.; Takano, K.; Murakami, S.; Matsumura, H.; Inoue, T.; et al. Growth of high-quality metastable crystal of acetaminophen using solution-mediated phase transformation at low supersaturation. J. Cryst. Growth 2018, 502, 76–82. [Google Scholar] [CrossRef]
- Fujimoto, R.; Maruyama, M.; Okada, S.; Adachi, H.; Yoshikawa, H.; Takano, K.; Imanishi, M.; Tsukamoto, K.; Yoshimura, M.; Mori, Y. Large-scale crystallization of acetaminophen trihydrate by a novel stirring technique. Appl. Phys. Express 2019, 12, 045503. [Google Scholar] [CrossRef]
- Nishigaki, A.; Maruyama, M.; Numata, M.; Kanzaki, C.; Tanaka, S.-I.; Yoshikawa, H.; Imanishi, M.; Yoshimura, M.; Mori, Y.; Takano, K. Microflow system promotes acetaminophen crystal nucleation. Eng. Life Sci. 2020, 20, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Tsuri, Y.; Inaoka, N.; Maruyama, M.; Adachi, H.; Yoshikawa, H.Y.; Takano, K.; Tsukamoto, K.; Imanishi, M.; Yoshimura, M.; Mori, Y. Effects of entrapped gas on the surface of a plastic ball induced by ultrasonic irradiation on the enhancement of crystallization of acetaminophen form II. J. Cryst. Growth 2021, 557, 125994. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Mattisson, C.; Roger, P.; Jönsson, B.; Axelsson, A.; Zacchi, G. Diffusion of lysozyme in gels and liquids. A general approach for the determination of diffusion coefficients using holographic laser interferometry. J. Chromatogr. B Biomed. Sci. Appl. 2000, 743, 151–167. [Google Scholar] [CrossRef]
- Tanaka, S.; Ito, K.; Hayakawa, R. Size and number density of precrystalline aggregates in lysozyme crystallization process. J. Chem. Phys. 1999, 111, 10330. [Google Scholar] [CrossRef]
- Jin, X.; Chen, L.; Liu, Y.; Ye, T.; Hu, C.; Ren, J.; Chen, H.; Li, H. Patterning the internal structure of single crystals by gel incorporation. J. Phys. Chem. C 2019, 123, 13147–13153. [Google Scholar] [CrossRef]
- Shtukenberg, A.G.; Ward, M.D.; Kahr, B. Crystal growth with macromolecular additives. Chem. Rev. 2017, 117, 14042–14090. [Google Scholar] [CrossRef]
- Jin, X.; Xue, J.; Kang, D.H.; Liu, Y.; Ren, J.; Wen, Q.; Hu, D.; Yu, Y.; Yang, W.; Du, B.; et al. Incorporation of fluorescent microgels inside calcite single crystals. Giant 2020, 3, 100023. [Google Scholar] [CrossRef]
- Liu, Y.; Zang, H.; Wang, L.; Fu, W.; Yuan, W.; Wu, J.; Jin, X.; Han, J.; Wu, C.; Wang, Y.; et al. Nanoparticles incorporated inside single-crystals: Enhanced fluorescent properties. Chem. Mater. 2016, 28, 7537–7543. [Google Scholar] [CrossRef]
- Kahr, B.; Gurney, R.W. Dyeing crystals. Chem. Rev. 2001, 101, 893–952. [Google Scholar] [CrossRef] [PubMed]
- Gurney, R.W.; Mitchell, C.A.; Ham, S.; Bastin, L.D.; Kahr, B. Salting benzenes. J. Phys. Chem. B 2000, 104, 878–892. [Google Scholar] [CrossRef]
- Sugiyama, S.; Hirose, M.; Shimizu, N.; Niiyama, M.; Maruyama, M.; Sazaki, G.; Murai, R.; Adachi, H.; Takano, K.; Murakami, S.; et al. Effect of evaporation on protein crystals grown in semi-solid agarose hydrogel. Jpn. J. Appl. Phys. 2011, 50, 025502. [Google Scholar] [CrossRef]
- Murai, R.; Yoshikawa, H.Y.; Takahashi, Y.; Maruyama, M.; Sugiyama, S.; Sazaki, G.; Adachi, H.; Takano, K.; Matsumura, H.; Murakami, S.; et al. Enhancement of femtosecond laser-induced nucleation of protein in a gel solution. Appl. Phys. Lett. 2010, 96, 043702. [Google Scholar] [CrossRef] [Green Version]
- Berg, W.F. Crystal growth from solutions. Proc. R. Soc. A 1938, 164, 79–95. [Google Scholar]






| Form I | Form II | |||
|---|---|---|---|---|
| Agarose concentration (%(w/v)) | 0 | 1.0 | 0 | 1.0 |
| Solubility (mg/mL) | 24.5 | 24.6 | 25.7 | 25.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishigaki, A.; Maruyama, M.; Tanaka, S.-i.; Yoshikawa, H.Y.; Imanishi, M.; Yoshimura, M.; Mori, Y.; Takano, K. Growth of Acetaminophen Polymorphic Crystals and Solution-Mediated Phase Transition from Trihydrate to Form II in Agarose Gel. Crystals 2021, 11, 1069. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11091069
Nishigaki A, Maruyama M, Tanaka S-i, Yoshikawa HY, Imanishi M, Yoshimura M, Mori Y, Takano K. Growth of Acetaminophen Polymorphic Crystals and Solution-Mediated Phase Transition from Trihydrate to Form II in Agarose Gel. Crystals. 2021; 11(9):1069. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11091069
Chicago/Turabian StyleNishigaki, Akari, Mihoko Maruyama, Shun-ichi Tanaka, Hiroshi Y. Yoshikawa, Masayuki Imanishi, Masashi Yoshimura, Yusuke Mori, and Kazufumi Takano. 2021. "Growth of Acetaminophen Polymorphic Crystals and Solution-Mediated Phase Transition from Trihydrate to Form II in Agarose Gel" Crystals 11, no. 9: 1069. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11091069