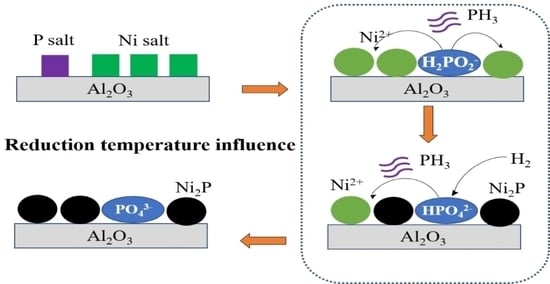
Influence of Reduction Temperature on the Structure and Naphthalene Hydrogenation Saturation Performance of Ni2P/Al2O3 Catalysts
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization of the Catalysts
3.1.1. Crystalline Structure
3.1.2. Morphology and Particle Size
3.1.3. Electronic Properties
3.1.4. Acidic Analysis
3.1.5. Textural and Structural Property
3.2. Naphthalene Hydrogenation Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoon, E.M.; Selvaraj, L.; Song, C.; Stallman, J.B.; Coleman, M.M. High-temperature stabilizers for jet fuels and similar hydrocarbon mixtures. 1. Comparative studies of hydrogen donors. Energy Fuel 1996, 10, 806–811. [Google Scholar] [CrossRef]
- Zhang, X.W.; Pan, L.; Wang, L.; Zou, J.J. Review on synthesis and properties of high-energy-density liquid fuels: Hydrocarbons, nanofluids and energetic ionic liquids. Chem. Eng. Sci. 2018, 180, 95–125. [Google Scholar] [CrossRef]
- Jia, T.h.; Zhang, X.w.; Liu, Y.; Gong, S.; Deng, C.; Pan, L.; Zou, J.J. A comprehensive review of the thermal oxidation stability of jet fuels. Chem. Eng. Sci. 2021, 229, 116157. [Google Scholar] [CrossRef]
- Beltramone, A.R.; Resasco, D.E.; Alvarez, W.E.; Choudhary, T.V. Simultaneous hydrogenation of multiring aromatic compounds over NiMo Catalyst. Ind. Eng. Chem. Res. 2008, 47, 7161–7166. [Google Scholar] [CrossRef]
- Fu, W.Q.; Zhang, L.; Wu, D.F.; Xiang, M.; Zhuo, Q.; Huang, K.; Tao, Z.D.; Tang, T.D. Mesoporous zeolite-supported metal sulfide catalysts with high activities in the deep hydrogenation of phenanthrene. J. Catal. 2015, 330, 423–433. [Google Scholar] [CrossRef]
- Badoga, S.; Dalai, A.K.; Adjaye, J.; Hu, Y. Combined effects of EDTA and heteroatoms (Ti, Zr, and Al) on catalytic activity of SBA-15 supported NiMo catalyst for hydrotreating of heavy gas oil. Ind. Eng. Chem. Res. 2014, 53, 2137–2156. [Google Scholar] [CrossRef]
- Du, M.X.; Qin, Z.F.; Ge, H.; Li, X.K.; Lü, Z.J.; Wang, J.G. Enhancement of Pd–Pt/Al2O3 catalyst performance in naphthalene hydrogenation by mixing different molecular sieves in the support. Fuel Process. Technol. 2010, 91, 1655–1661. [Google Scholar] [CrossRef]
- Pinilla, J.L.; Garcia, A.B.; Philippot, K.; Lara, P.; Garcia-Suarez, E.J.; Millan, M. Carbon-supported Pd nanoparticles as catalysts for anthracene hydrogenation. Fuel 2014, 116, 729–735. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Lv, G.; Zhang, L. Novel method to synthesize Ni2P/SBA-15 adsorbents for the adsorptive desulfurization of model diesel fuel. J. Alloys Compd. 2018, 745, 467–476. [Google Scholar] [CrossRef]
- Song, H.; Wang, J.; Wang, Z.D.; Song, H.L.; Li, F.; Jin, Z.S. Effect of titanium content on dibenzothiophene HDS performance over Ni2P/Ti-MCM-41 catalyst. J. Catal. 2014, 311, 257–265. [Google Scholar] [CrossRef]
- Fang, M.; Tang, W.; Yu, C.; Xia, L.; Xia, Z.; Wang, Q.; Luo, Z. Performance of Ni-rich bimetallic phosphides on simultaneous quinoline hydrodenitrogenation and dibenzothiophene hydrodesulfurization. Fuel Process. Technol. 2015, 129, 236–244. [Google Scholar] [CrossRef]
- Tian, S.; Li, X.; Wang, A.; Chen, y.; Li, H.; Hu, Y. Hydrodenitrogenation of quinoline and decahydroquinoline over a surface nickel phosphosulfide phase. Catal. Lett. 2018, 148, 1579–1588. [Google Scholar] [CrossRef]
- Yun, G.; Guan, Q.; Li, W. Nondestructive construction of Lewis acid sites on the surface of supported nickel phosphide catalysts by atomic-layer deposition. J. Catal. 2018, 361, 12–22. [Google Scholar] [CrossRef]
- Hu, D.; Duan, A.; Xu, C.; Zheng, P.; Li, Y.; Xiao, C.; Liu, C.; Meng, Q.; Li, H. Ni2P promotes the hydrogenation activity of naphthalene on wrinkled silica nanoparticles with tunable hierarchical pore sizes in a large range. NanoScale 2019, 11, 15519–15529. [Google Scholar] [CrossRef] [PubMed]
- Oyama, S.T.; Lee, Y.K. The active site of nickel phosphide catalysts for the hydrodesulfurization of 4,6-DMDBT. J. Catal. 2008, 258, 393–400. [Google Scholar] [CrossRef]
- Vargas-Villagrán, H.; Ramírez-Suárez, D.; Ramírez-Muñoz, G.; Calzada, L.A.; González-García, G.; Klimova, T.E. Tuning of activity and selectivity of Ni/(Al)SBA-15 catalysts in naphthalene hydrogenation. Catal. Today 2021, 360, 27–37. [Google Scholar] [CrossRef]
- Tan, Q.; Cao, Y.; Li, J. Prepared multifunctional catalyst Ni2P/Zr-SBA-15 and catalyzed Jatropha Oil to producebio-aviation fuel. Renew. Energy 2020, 150, 370–381. [Google Scholar] [CrossRef]
- Jing, J.Y.; Wang, J.Z.; Liu, D.C.; Qie, Z.Q.; Bai, H.C.; Li, W.Y. Naphthalene hydrogenation saturation over Ni2P/Al2O3 catalysts synthesized by thermal decomposition of hypophosphite. ACS Omega 2020, 5, 31423–31431. [Google Scholar] [CrossRef]
- Jing, J.Y.; Yang, Z.F.; Wang, J.Z.; Liu, D.C.; Feng, J.; Li, W.Y. Effect of preparation methods on the structure and naphthalene hydrogenation performance of Ni2P/SiO2 catalyst. J. Fuel Chem. Technol. 2020, 48, 842–851. [Google Scholar] [CrossRef]
- Hédoire, C.E.; Louis, C.; Davidson, A.; Breysse, M.; Maugé, F.; Vrinat, M.; Hédoire, C.E.; Louis, C.; Davidson, A.; Breysse, M. Support effect in hydrotreating catalysts: Hydrogenation properties of molybdenum sulfide supported on β-zeolites of various acidities. J. Catal. 2003, 220, 433–441. [Google Scholar] [CrossRef]
- Lan, X.; Hensen, E.J.M.; Weber, T. Silica-supported Ni2P: Effect of preparation conditions on structure and catalytic performance in thiophene hydrodesulfurization (HDS). Catal. Today 2017, 292, 121–132. [Google Scholar] [CrossRef]
- Oyama, S.T.; Gott, T.; Zhao, H.; Lee, Y.-K. Transition metal phosphide hydroprocessing catalysts: A review. Catal. Today 2009, 143, 94–107. [Google Scholar] [CrossRef]
- Guan, Q.; Li, W. A novel synthetic approach to synthesizing bulk and supported metal phosphides. J. Catal. 2010, 271, 413–415. [Google Scholar] [CrossRef]
- Wu, H.; Ni, Y.; Wang, M.; Lu, D. Shape-controlled synthesis and performance comparison of Ni2P nanostructures. CrystEngComm 2016, 18, 5155–5163. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Chen, G.; He, Z. Highly loaded and dispersed Ni2P/Al2O3 catalyst with high selectivity for hydrogenation of acetophenone. Catalysts 2018, 8, 309. [Google Scholar] [CrossRef] [Green Version]
- Chu, S.; Li, X.; Zhou, X.R.; Prins, R.; Waqas, Q.; Wang, A.J.; Sheng, Q.; Hao, Q.L. Preparation of Ni2P supported on Al2O3 and B2O3 mixed oxides by yemperature-programmed reduction of phosphate precursors withlLow P/Ni ratios. Top. Catal. 2020, 63, 1379–1387. [Google Scholar] [CrossRef]
- Bussell, M.E.; Miles, C.E.; Carlson, T.R.; Morgan, B.J.; Topalian, P.J.; Schare, J.R. Hydrodesulfurization properties of nickel phosphide on borontreated alumina supports. ChemCatChem 2020, 12, 4939–4950. [Google Scholar]
- Dang, Y.; Liu, Y.B.; Feng, X.; Chen, X.B.; Yang, C.H. Effect of dispersion on the adsorption of polycyclic aromatic hydrocarbons over the γ-Al2O3 (110) surface. Appl. Surf. Sci. 2019, 486, 137–143. [Google Scholar] [CrossRef]





| Catalyst | Niδ+ Peak Area | Pδ− Peak Area | Niδ+ area Percentage (%) | Pδ− Area Percentage (%) |
|---|---|---|---|---|
| Cat-300 | 6053 | 214 | 9.95 | 11.29 |
| Cat-350 | 7256 | 507 | 11.67 | 18.40 |
| Cat-400 | 9506 | 519 | 32.91 | 28.66 |
| Cat-450 | 3735 | 257 | 15.72 | 12.47 |
| Cat-500 | 2450 | 209 | 11.03 | 12.67 |
| Catalysts | Bronsted Acidity Value (μmol g−1) | Lewis Acidity Value (μmol g−1) |
|---|---|---|
| Cat-300 | 3.6 | 190.3 |
| Cat-350 | 3.3 | 180.3 |
| Cat-400 | 0.4 | 158.2 |
| Cat-450 | 0.2 | 61.8 |
| Cat-500 | 0.1 | 53.4 |
| Al2O3 | 0 | 131.8 |
| Catalyst | Specific Surface Area (m2 g−1) | Pore Size (nm) | Particle Size a (nm) | CO Uptake (μmol g−1) |
|---|---|---|---|---|
| Cat-300 | 134 | 2.8 | 3.2 | 13 |
| Cat-350 | 163 | 3.8 | 3.4 | 19 |
| Cat-400 | 170 | 5.2 | 3.8 | 30 |
| Cat-450 | 159 | 4.3 | 5.1 | 22 |
| Cat-500 | 158 | 3.4 | 7.2 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Jing, J.-Y.; Qie, Z.-Q.; Li, W.-Y. Influence of Reduction Temperature on the Structure and Naphthalene Hydrogenation Saturation Performance of Ni2P/Al2O3 Catalysts. Crystals 2022, 12, 318. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12030318
Li Z, Jing J-Y, Qie Z-Q, Li W-Y. Influence of Reduction Temperature on the Structure and Naphthalene Hydrogenation Saturation Performance of Ni2P/Al2O3 Catalysts. Crystals. 2022; 12(3):318. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12030318
Chicago/Turabian StyleLi, Ze, Jie-Ying Jing, Zhi-Qiang Qie, and Wen-Ying Li. 2022. "Influence of Reduction Temperature on the Structure and Naphthalene Hydrogenation Saturation Performance of Ni2P/Al2O3 Catalysts" Crystals 12, no. 3: 318. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12030318