Synthesis of Furan-Substituted N-Heteroacene-Based Liquid Material and Its Acid-Recognizing Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Synthesis of 6,7-bis(3,7,11-trimethyl-1-dodecyloxy)-2,3-difurylquinoxaline (RPNL 1)
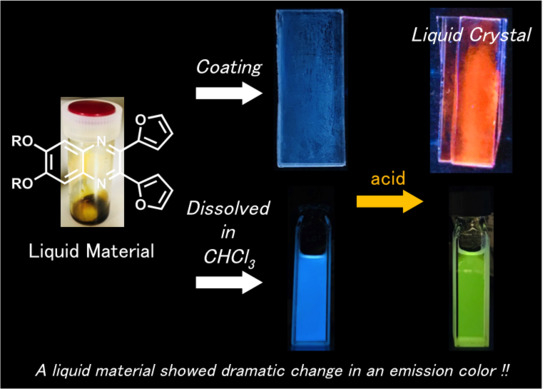
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References and Note
- Chen, L.-J.; Yang, H.-B. Construction of Stimuli-Responsive Functional Materials Via Hierarchical Self-Assembly Involving Coordination Interactions. Acc. Chem. Res. 2018, 51, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Isapour, G.; Lattuada, M. Bioinspired Stimuli-Responsive Color-Changing Systems. Adv. Mater. 2018, 30, 1707069. [Google Scholar] [CrossRef] [PubMed]
- Montero de Espinosa, L.; Meesorn, W.; Moatsou, D.; Weder, C. Bioinspired Polymer Systems with Stimuli-Responsive Mechanical Properties. Chem. Rev. 2017, 117, 12851–12892. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ji, X.; Li, Z.; Huang, F. Fluorescent Supramolecular Polymeric Materials. Adv. Mater. 2017, 29, 1606117. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, F.; Zheng, B.; Huang, F. Stimuli-Responsive Supramolecular Polymeric Materials. Chem. Soc. Rev. 2012, 41, 6042–6065. [Google Scholar] [CrossRef]
- Löwe, C.; Weder, C. Oligo(P-Phenylene Vinylene) Excimers as Molecular Probes: Deformation-Induced Color Changes in Photoluminescent Polymer Blends. Adv. Mater. 2002, 14, 1625–1629. [Google Scholar] [CrossRef]
- Mutai, T.; Satou, H.; Araki, K. Reproducible on-Off Switching of Solid-State Luminescence by Controlling Molecular Packing through Heat-Mode Interconversion. Nat. Mater. 2005, 4, 685–687. [Google Scholar] [CrossRef]
- Qi, Z.; Schalley, C.A. Exploring Macrocycles in Functional Supramolecular Gels: From Stimuli Responsiveness to Systems Chemistry. Acc. Chem. Res. 2014, 47, 2222–2233. [Google Scholar] [CrossRef]
- Sagara, Y.; Kato, T. Mechanically Induced Luminescence Changes in Molecular Assemblies. Nat. Chem. 2009, 1, 605–610. [Google Scholar] [CrossRef]
- Sagara, Y.; Mutai, T.; Yoshikawa, I.; Araki, K. Material Design for Piezochromic Luminescence: Hydrogen-Bond-Directed Assemblies of a Pyrene Derivative. J. Am. Chem. Soc. 2007, 129, 1520–1521. [Google Scholar] [CrossRef]
- Santhosh, B.S.; Aimi, J.; Ozawa, H.; Shirahata, N.; Saeki, A.; Seki, S.; Ajayaghosh, A.; Mohwald, H.; Nakanishi, T. Solvent-Free Luminescent Organic Liquids. Angew. Chem. Int. Ed. 2012, 51, 3391–3395. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, B.S.; Hollamby, M.J.; Aimi, J.; Ozawa, H.; Saeki, A.; Seki, S.; Kobayashi, K.; Hagiwara, K.; Yoshizawa, M.; Mohwald, H.; et al. Nonvolatile Liquid Anthracenes for Facile Full-Colour Luminescence Tuning at Single Blue-Light Excitation. Nat. Commun. 2013, 4, 1969. [Google Scholar]
- Isoda, K.; Sato, Y.; Matsukuma, D. Fluorescent N-Heteroacene-Based π-Conjugated Liquid Responsive to Hcl Vapor. Chem. Select. 2017, 2, 7222–7226. [Google Scholar] [CrossRef]
- Lu, F.; Takaya, T.; Iwata, K.; Kawamura, I.; Saeki, A.; Ishii, M.; Nagura, K.; Nakanishi, T. A Guide to Design Functional Molecular Liquids with Tailorable Properties Using Pyrene-Fluorescence as a Probe. Sci. Rep. 2017, 7, 3416. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Jang, K.; Osica, I.; Hagiwara, K.; Yoshizawa, M.; Ishii, M.; Chino, Y.; Ohta, K.; Ludwichowska, K.; Kurzydłowski, K.J.; et al. Supercooling of Functional Alkyl-Π Molecular Liquids. Chem. Sci. 2018, 9, 6774–6778. [Google Scholar] [CrossRef]
- Lu, F.; Kitamura, N.; Takaya, T.; Iwata, K.; Nakanishi, T.; Kurashige, Y. Experimental and Theoretical Investigation of Fluorescence Solvatochromism of Dialkoxyphenyl-Pyrene Molecules. Phys. Chem. Chem. Phys. 2018, 20, 3258–3264. [Google Scholar] [CrossRef]
- Narayan, B.; Nagura, K.; Takaya, T.; Iwata, K.; Shinohara, A.; Shinmori, H.; Wang, H.; Li, Q.; Sun, X.; Li, H.; et al. The Effect of Regioisomerism on the Photophysical Properties of Alkylated-Naphthalene Liquids. Phys. Chem. Chem. Phys. 2018, 20, 2970–2975. [Google Scholar] [CrossRef]
- Sato, Y.; Mutoh, Y.; Matsukuma, D.; Nakagawa, M.; Kawai, T.; Isoda, K. Tuning the Electronic Properties and Acid-Response Behavior of N-Heteroacene-Based π-Conjugated Liquids by Changing the Number of Π-Conjugated Substituents. Chem. Asian J. 2018, 13, 2619–2625. [Google Scholar] [CrossRef]
- Isoda, K.; Nakamura, M.; Tatenuma, T.; Ogata, H.; Sugaya, T.; Tadokoro, M. Synthesis and Characterization of Electron-Accepting Nonsubstituted Tetraazaacene Derivatives. Chem. Lett. 2012, 41, 937–939. [Google Scholar] [CrossRef]
- Bunz, U.H.F.; Engelhart, J.U.; Lindner, B.D.; Schaffroth, M. Large N-Heteroacenes: New Tricks for Very Old Dogs? Angew. Chem. Int. Ed. 2013, 52, 3810–3821. [Google Scholar] [CrossRef]
- Isoda, K.; Abe, T.; Tadokoro, M. Room-Temperature Redox-Active Liquid Crystals Composed of Tetraazanaphthacene Derivatives. Chem. Asian J. 2013, 8, 2951–2954. [Google Scholar] [CrossRef] [PubMed]
- Isoda, K.; Abe, T.; Funahashi, M.; Tadokoro, M. Electron Transport of Photoconductive n-Type Liquid Crystals Based on a Redox-Active Tetraazanaphthacene Framework. Chem. Eur. J. 2014, 20, 7232–7235. [Google Scholar] [CrossRef] [PubMed]
- Bunz, U.H.F. The Larger Linear N-Heteroacenes. Acc. Chem. Res. 2015, 48, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Isoda, K.; Abe, T.; Kawamoto, I.; Tadokoro, M. Self-Organized Superstructure and Electronic Properties of a Liquid-Crystalline Tetraazapentacene Derivative. Chem. Lett. 2015, 44, 126–128. [Google Scholar] [CrossRef]
- Isoda, K. Acid-Responsive N-Heteroacene-Based Material Showing Multi-Emission Colors. ChemistryOpen 2017, 6, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, P.C.; Pople, J.A. The Effect of D-Functions on Molecular Orbital Energies for Hydrocarbons. Chem. Phys. Lett. 1972, 16, 217–219. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta. 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Bondi, A. Van Der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Yoshio, M.; Mukai, T.; Ohno, H.; Kato, T. One-Dimensional Ion Transport in Self-Organized Columnar Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 994–995. [Google Scholar] [CrossRef]
- The average number of molecules in a column stratum (μ) was estimated according to the following equation: μ = (NAabhρ)/M, in which NA is Avogadro’s number, a and b is the lattice parameter (a = 42.1 Å and b = 38.4 Å), h is the thickness of the stratum (estimated to be ca. 3.7 Å, based on XRD analysis), ρ is the density (assumed to be 1 g cm−3), and M is te molecular weight of RPNL 1·BSA.





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isoda, K.; Ikenaga, A. Synthesis of Furan-Substituted N-Heteroacene-Based Liquid Material and Its Acid-Recognizing Behavior. Crystals 2019, 9, 51. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9010051
Isoda K, Ikenaga A. Synthesis of Furan-Substituted N-Heteroacene-Based Liquid Material and Its Acid-Recognizing Behavior. Crystals. 2019; 9(1):51. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9010051
Chicago/Turabian StyleIsoda, Kyosuke, and Ayumi Ikenaga. 2019. "Synthesis of Furan-Substituted N-Heteroacene-Based Liquid Material and Its Acid-Recognizing Behavior" Crystals 9, no. 1: 51. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9010051