Synthesis of MgFe Layered Double Hydroxide from Iron-Containing Acidic Residual Solution and Its Adsorption Performance
Abstract
:1. Introduction
2. Experimental
2.1. Mg/Fe–LDH Synthesis and Characterization
2.2. Adsorption Studies
3. Results and Discussions
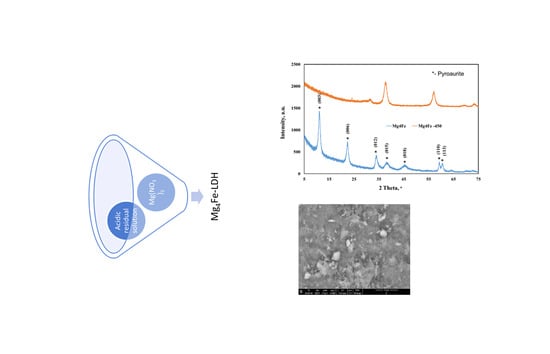
3.1. Material Characterizations
3.2. Equilibrium Studies
3.3. Kinetic Studies
3.4. Thermodynamic Studies
3.5. Adsorption Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dore, E.; Frau, F.; Cidu, R. Antimonate Removal from Polluted Mining Water by Calcined Layered Double Hydroxides. Crystals 2019, 9, 410. [Google Scholar] [CrossRef]
- Cocheci, L.; Lupa, L.; Lazau, R.; Voda, R.; Pode, R. Zinc recovery from waste zinc ash—A new “green” route for the preparation of Zn-Al layered double hydroxide used for molybdate retention. J. Alloys Compd. 2019, 787, 332–343. [Google Scholar] [CrossRef]
- Lupa, L.; Cocheci, L.; Pode, R.; Hulka, I. Phenol adsorption using Aliquat 336 functionalized Zn-Al layered double hydroxide. Sep. Pur. Technol. 2018, 196, 82–95. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Shamsayei, M.; Tahmasebi, E. Highly selective and efficient removal and extraction of heavy metals by layered double hydroxides intercalated with the diphenylamine-4-sulfonate: A comparative study. Chem. Eng. J. 2017, 323, 212–223. [Google Scholar] [CrossRef] [Green Version]
- Carriazo, D.; del Arco, M.; Martin, C.; Rives, V. A comparative study between chloride and calcined carbonate hydrotalcites as adsorbents for Cr (VI). Appl. Clay Sci. 2007, 37, 231–239. [Google Scholar] [CrossRef]
- Ardau, C.; Frau, F.; Dore, E.; Lattanzi, P. Molybdate sorption by Zn-Al sulphate layered double hydroxides. Appl. Clay Sci. 2012, 65–66, 128–133. [Google Scholar] [CrossRef]
- Koilraj, P.; Srinivasan, K. ZnAl layered double hydroxides as potential molybdate sorbents and valorise the exchanged sorbent for catalytic wet peroxide oxidation of phenol. Ind. Eng. Chem. Res. 2013, 52, 7373–7381. [Google Scholar] [CrossRef]
- Ashekuzzaman, S.M.; Jiang, J.Q. Strategic phosphate removal/recovery by a re-usable Mg-Fe-Cl layered double hydroxide. Process. Saf. Environ. Prot. 2017, 107, 454–462. [Google Scholar] [CrossRef]
- Wan, S.; Wang, S.; Li, Y.; Gao, B. Functionalizing biochar with Mg-Al and Mg-Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 2017, 47, 246–253. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Kishida, M.; Nakamura, T.; Kawasaki, N. Interaction between phosphate ions and Fe-Mg type hydrotalcite for purification of wastewater. J. Environ. Chem. Eng. 2019, 7, 102897. [Google Scholar] [CrossRef]
- Matsuik, J.; Rybka, K. Removal of chromates and sulphates by Mg/Fe LDH and heterostructured LDH/Halloysite materials: Efficiency, selectivity, and stability of adsorbents in single and multi-element systems. Materials 2019, 12, 1373. [Google Scholar] [CrossRef] [PubMed]
- Caporale, A.G.; Pigna, M.; Azam, S.M.G.G.; Sommella, A.; Rao, M.A.; Violante, A. Effect of competing ligands on the sorption/desorption of arsenite on/from Mg-Fe layered double hydroxides (Mg-Fe-LDH). Chem. Eng. J. 2013, 225, 704–709. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, X.; Wu, Z.; Zeng, G.; Jiang, L.; Peng, X.; Li, H. Adsorption behaviour and mechanism of Mg/Fe layered double hydroxide with Fe3O4-carbon sphere on the removal of Pb(II) and Cu(II). J. Coll. Interf. Sci. 2019, 536, 440–455. [Google Scholar] [CrossRef]
- Das, J.; Sairam Patra, B.; Baliarsingh, N.; Parida, K.M. Calcined Mg-Fe-CO3 LDH as and adsorbent for the removal of selenite. J. Coll. Interf. Sci. 2007, 316, 216–223. [Google Scholar] [CrossRef]
- Hudcova, B.; Erben, M.; Vitkova, M.; Komarek, M. Antimonate adsorption onto Mg-Fe layered double hydroxides in aqueous solutions at different pH values: Coupling surface complexation modelling with solid-state analyses. Chemosphere 2019, 229, 236–246. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Gasser, M.S. Adsorption study of anionic reactive dye from aqueous solution to Mg-Fe-CO3 layered double hydroxides (LDH). Appl. Surf. Sci. 2012, 259, 650–656. [Google Scholar] [CrossRef]
- Ayawei, N.; Angaye, S.S.; Wankasi, D. Mg/Fe layered double hydroxide as a novel adsorbent for the removal of Congo red. Int. J. Appl. Sci. Technol. 2017, 7, 83–92. [Google Scholar]
- Abdelkader, N.B.-H.; Bentouami, A.; Derriche, Z.; Bettahar, N.; de Ménorval, L.-C. Synthesis and characterization of Mg–Fe layer double hydroxides and its application on adsorption of Orange G from aqueous solution. Chem. Eng. J. 2011, 169, 231–238. [Google Scholar] [CrossRef]
- Zhang, S.; Jiao, Q.; Wang, C.; Yu, H.; Zhao, Y.; Li, H.; Wu, Q. In situ synthesis of Mg/Fe LDO/carbon nanohelix composites as adsorbing materials. J. All. Comp. 2016, 658, 505–512. [Google Scholar] [CrossRef]
- Tahir, N.; Abdelssadek, Z.; Halliche, D.; Saadi, A.; Chebout, R.; Cherifi, O.; Bachari, K. Mg-Fe-hydrotalcite as catalyst for the benzylation of benzene and other aromatics by benzyl chloride reactions. Surf. Inter. Anal. 2008, 40, 254–258. [Google Scholar] [CrossRef]
- Chang, P.S.; Li, S.Y.; Juang, T.Y.; Liu, Y.C. Mg-Fe layered double hydroxides enhance surfactin production in bacterial cells. Crystals 2019, 9, 355. [Google Scholar] [CrossRef]
- Muriithi, G.N.; Petrik, L.F.; Gitari, W.M.; Doucet, F.J. Synthesis and characterization of hydrotalcite from South Africa Coal fly ash. Powder Technol. 2017, 312, 299–309. [Google Scholar] [CrossRef]
- Cocheci, L.; Lupa, L.; Gheju, M.; Golban, A.; Lazau, R.; Pode, R. Zn–Al–CO3 layered double hydroxides prepared from a waste of hot-dip galvanizing process. Clean Technol. Environ. Policy 2018, 20, 1105–1112. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Yamashita, H. Synthesis of Ca-based layered double hydroxide from blast furnace slag and its catalytic applications. ISIJ Intern. 2015, 7, 1531–1537. [Google Scholar] [CrossRef]
- Galindo, R.; Lopez-Delgato, A.; Padilla, I.; Yates, M. Hydrotalcite-like compounds: A way to recover a hazardous waste in the aluminium tertiary industry. Appl. Clay Sci. 2014, 95, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Murayama, N.; Maekawa, I.; Ushiro, H.; Miyoshi, T.; Shibata, J.; Valix, M. Synthesis of various layered double hydroxides using aluminium dross generated in aluminium recycling process. Int. J. Miner. Process. 2012, 110–111, 46–52. [Google Scholar] [CrossRef]
- Golban, A.; Cocheci, L.; Lazau, R.; Lupa, L.; Pode, R. Iron ions reclaiming from sludge resulted from hot-dip galvanizing process, as Mg3Fe-layered double hydroxide used in the degradation process of organic dyes. Des. Water Treat. 2018, 131, 317–327. [Google Scholar] [CrossRef]
- Panayotova, M.; Panayotov, V. An Electrochemical Method for Decreasing the Concentration of Sulfate and Molybdenum Ions in Industrial Wastewater. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2004, 39, 173–183. [Google Scholar] [CrossRef]
- Lievens, P.; Block, C.; Cornelis, G.; Vandecasteele, C.; De Voogd, J.C.; Van Brecht, A. Mo, Sb and Se Removal from Scrubber Effluent of a Waste Incinerator. In Water Treatment Technologies for the Removal of High-Toxicity Pollutants; Vaclavikova, M., Vitale, K., Gallios, G.P., Ivanicova, L., Eds.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Verbinnen, B.; Block, C.; Hannes, D.; Lievens, P.; Vaclavikova, M.; Stefusova, K.; Gallios, G.; Vandecasteele, C. Removal of molybdate anions from water by adsorption on zeolite-supported magnetite. Water Environ. Res. 2012, 84, 753–760. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, Fourth Edition. 2011. Available online: http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf (accessed on 2 September 2019).
- Underwood, E.J. Trace Elements in Human and Animal Nutrition, 3rd ed.; Academic Press: London, UK, 1971. [Google Scholar]
- Palmer, S.J.; Soisonard, A.; Frost, R.I. Determination of the mechanism(s) for the inclusion of arsenate, vanadate or molybdate anions into hydrotalcites with variable cationic ratio. J. Colloid Interface Sci. 2009, 329, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Tkac, P.; Paulenova, A. Speciation of molybdenum (VI) in aqueous and organic phases of selected extraction systems. Sep. Sci. Technol. 2008, 43, 2641–2657. [Google Scholar] [CrossRef]
- Pietrelli, L.; Ferro, S.; Vocciante, M. Raw materials recovery from spent hydrochloric acid-based galvanizing wastewater. Chem. Eng. J. 2018, 341, 539–546. [Google Scholar] [CrossRef]
- Ferreira, O.P.; Alves, O.L.; Gouveia, D.X.; Souza Filho, A.G.; de Paiva, J.A.C.; Mendes Filho, J. Thermal decomposition and structural Reconstruction effect on Mg–Fe based hydrotalcite compounds. J. Solid State Chem. 2004, 177, 3058–3069. [Google Scholar] [CrossRef]
- Elmoubarki, R.; Mahjoubi, F.Z.; Elhalil, A.; Tounsadi, H.; Abdennouri, M.; Sadiq, M.; Qourzal, S.; Zouhri, A.; Barka, N. Ni/Fe and Mg/Fe layered double hydroxides and their calcined derivatives: Preparation, characterization and application on textile dyes removal. J. Mat. Res. Technol. 2017, 6, 271–283. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR-JAC 2012, 3, 38–45. [Google Scholar]
- Ertugay, N.; Malkoc, E. Adsorption isotherm, kinetic, and thermodynamic studies for methylene blue from aqueous solution by needles of Pinus sylvestris L. Pol. J. Environ. Stud. 2014, 23, 1995–2006. [Google Scholar]
- Zhang, L.; Huang, T.; Zhang, M.; Guo, X.; Yuan, Z. Studies on the capability and behavior of adsorption of thallium on nano-Al2O3. J. Hazard. Mater. 2008, 157, 352–357. [Google Scholar] [CrossRef]
- Paikaray, S.; Hendry, M.J.; Essilfie-Dughan, J. Controls on arsenate, molybdate, and selenate uptake by hydrotalcite-like layered double hydroxides. Chem. Geol. 2013, 345, 130–138. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Roobottom, H.K.; Passmore, J.; Glasser, L. Relationships among ionic lattice energies, molecular (formula unit) volumes, and thermochemical radii. Inorg. Chem. 1999, 38, 3609–3620. [Google Scholar] [CrossRef]
- Smith, H.D.; Parkinson, G.M.; Hart, R.D. In situ absorption of molybdate and vanadate during precipitation of hydrotalcite from sodium aluminate solutions. J. Cryst. Grow. 2005, 275, e1665–e1671. [Google Scholar] [CrossRef]













| No. | Parameter | Value |
|---|---|---|
| 1. | pH | 2.15 |
| 2. | Fe | 65.0 g/L |
| 3. | Cu | 57.4 mg/L |
| 4. | Cd | 0.453 mg/L |
| 5. | Pb | 15.2 mg/L |
| 6. | Zn | 565 mg/L |
| 7. | Cl− | 81.7 g/L |
| 8. | TOC | 68 mg/L |
| Parameters | Mg4Fe | Mg4Fe–450 | |
|---|---|---|---|
| Molar ratio | Theoretical | 4 | 4 |
| Experimental | 3.98 | 4.08 | |
| SBET (m2/g) | 101 | 0.536 | |
| Vp (cm3/g) | 192 | 0.802 | |
| Cd, % | 0.16 | 0.08 | |
| Pb, % | 0.12 | 0.22 | |
| Zn, % | 1.19 | 1.59 | |
| Type of Isotherm | Parameter | Adsorbent | |
|---|---|---|---|
| Mg4Fe | Mg4Fe-450 | ||
| Langmuir | KL, L/mg | 0.137 | 0.151 |
| qm calc, mg/g | 42.1 | 55.2 | |
| R2 | 0.9981 | 0.9964 | |
| Freundlich | KF, mg/g | 9.33 | 14.7 |
| 1/n | 0.3086 | 0.2908 | |
| R2 | 0.9112 | 0.9330 | |
| Temkin | KT, L/g | 1.006 | 1.016 |
| bT, J/mol | 875 | 758 | |
| R2 | 0.9286 | 0.9074 | |
| Dubinin–Radushkevich | Kad, mol2/kJ2 | 2 × 10−6 | 3 × 10−7 |
| qs, mg/g | 33.2 | 40.04 | |
| R2 | 0.7725 | 0.6644 | |
| Model | Parameter | Adsorbent | |||||
|---|---|---|---|---|---|---|---|
| Mg4Fe | Mg4Fe-450 | ||||||
| Temperature | Temperature | ||||||
| 20 °C | 35 °C | 50 °C | 20 °C | 35 °C | 50 °C | ||
| qe exp, mg/g | 31 | 32 | 32.5 | 35 | 35.5 | 36 | |
| Pseudo-first-order | K1, min−1 | 0.0176 | 0.0115 | 0.0142 | 0.0120 | 0.0194 | 0.0247 |
| qe calc, mg/g | 6.095 | 5.37 | 5.04 | 3.91 | 3.59 | 2.72 | |
| R2 | 0.8887 | 0.9189 | 0.9478 | 0.7772 | 0.6894 | 0.5104 | |
| Pseudo-second-order | K2, min/(mg/g) | 0.00934 | 0.0109 | 0.0119 | 0.0137 | 0.0163 | 0.0185 |
| qe calc, mg/g | 31.4 | 31.8 | 32.6 | 35 | 35.7 | 36.2 | |
| R2 | 0.9998 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | |
| Intraparticle diffusion | Kint | 1.082 | 0.909 | 0.874 | 1.051 | 1.038 | 1.004 |
| R2 | 0.9905 | 0.9777 | 0.9850 | 0.9356 | 0.8901 | 0.8780 | |
| Parameter | Adsorbent | ||
|---|---|---|---|
| Mg4Fe | Mg4Fe-450 | ||
| E, kJ/mol | 6.37 | 7.89 | |
| ΔHo, kJ/mol−1 | 3.35 | 2.14 | |
| ΔSo, J/(mol . K) | 15.3 | 14.4 | |
| R2 | 0.9869 | 0.9997 | |
| ΔGo, kJ/mol | 293 K 308 K 323 K | −1.13 | −2.15 |
| −1.36 | −2.30 | ||
| −1.59 | −2.51 | ||
| Adsorbent | qm mg/g | References |
|---|---|---|
| Zn3Al–CO3-LDH | 13.7 | [2] |
| Zn3Al-CLDH | 39.8 | |
| ZnAl3–Cl-LDH | 114.9 | [8] |
| ZnAl3–CO3-LDH | <10 | |
| MgFeSO4-type HT-LDH | 15.5 | [42] |
| Mg4Fe | 42.1 | Present paper |
| Mg4Fe-450 | 55.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golban, A.; Lupa, L.; Cocheci, L.; Pode, R. Synthesis of MgFe Layered Double Hydroxide from Iron-Containing Acidic Residual Solution and Its Adsorption Performance. Crystals 2019, 9, 514. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9100514
Golban A, Lupa L, Cocheci L, Pode R. Synthesis of MgFe Layered Double Hydroxide from Iron-Containing Acidic Residual Solution and Its Adsorption Performance. Crystals. 2019; 9(10):514. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9100514
Chicago/Turabian StyleGolban, Alin, Lavinia Lupa, Laura Cocheci, and Rodica Pode. 2019. "Synthesis of MgFe Layered Double Hydroxide from Iron-Containing Acidic Residual Solution and Its Adsorption Performance" Crystals 9, no. 10: 514. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9100514