2-Chloroalkoxy-Substituted Pentafluorinated Bistolanes as Novel Light-Emitting Liquid Crystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Materials
2.3. Typical Procedure for the Preparation of S-4a
2-[4-[2-(2-Chloropentyloxy)phenylethyn-1-yl]phenylethyn-1-yl]trimethylsilane (S-4a)
2.4. Typical Procedure for the Preparation of S-4b
2-[4-[2-(2-Chlorohexyloxy)phenylethyn-1-yl]phenylethyn-1-yl]trimethylsilane (S-4b)
2.5. Typical Procedure for the Preparation of S-5a
4-[2-(2-Chloropentyloxy)phenylethyn-1-yl]phenylacetylene (S-5a)
2.6. Typical Procedure for the Preparation of S-5b
4-[2-(2-Chlorohexyloxy)phenylethyn-1-yl]phenylacetylene (S-5b)
2.7. Typical Procedure for the Preparation of S-2a
1-[2-(2-Chloropentyloxy)phenylethyn-1-yl]-4-[2-(2,3,4,5,6-pentafluorophenyl)ethyn-1-yl]benzene (S-2a)
2.8. Typical Procedure for the Preparation of S-2b
1-[2-(2-Chlorohexyloxy)phenylethyn-1-yl]-4-[2-(2,3,4,5,6-bentafluorophenyl)ethyn-1-yl]benzene (S-2b)
2.9. X-Ray Crystallography
2.10. Phase Transition Behavior
2.11. Photophysical Behavior
2.12. Computation
3. Results and Discussion
3.1. Molecular Design, Synthesis, and Crystal Structure
3.2. Phase Transition Behavior
3.3. Photophysical Behavior in Solution
3.4. Photophysical Behavior in the Cr Phase
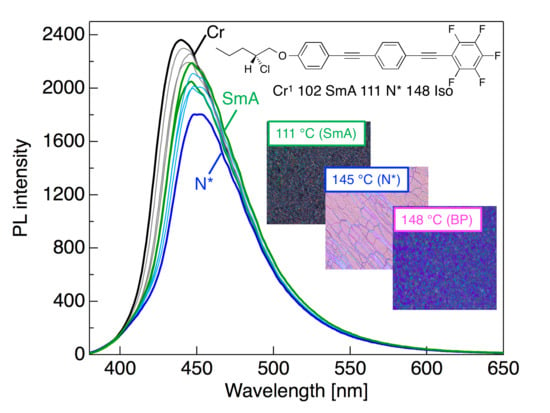
3.5. PL Behavior Through Phase Transition upon Heating and Cooling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, W.; Sun, Y.; Li, F. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Bui, T.T.; Goubard, F.; Ibrahim-Ouali, M.; Gigmes, D.; Dumur, F. Recent advances on organic blue thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs). Beilstein J. Org. Chem. 2018, 14, 282–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhulki, S.; Moorthy, J.N. Small molecular hole-transporting materials (HTMs) in organic light-emitting diodes (OLEDs): Structural diversity and classification. J. Mater. Chem. C 2018, 6, 8280–8325. [Google Scholar] [CrossRef]
- Hemmer, E.; Acosta-Mora, P.; Méndez-Ramos, J.; Fischer, S. Optical nanoprobes for biomedical applications: Shining a light on upconverting and near-infrared emitting nanoparticles for imaging, thermal sensing, and photodynamic therapy. J. Mater. Chem. B 2017, 5, 4365–4392. [Google Scholar] [CrossRef]
- Hou, J.-T.; Ren, W.X.; Li, K.; Seo, J.; Sharma, A.; Yu, X.-Q.; Kim, J.S. Fluorescent bioimaging of pH: From design to applications. Chem. Soc. Rev. 2017, 46, 2076–2090. [Google Scholar] [CrossRef]
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Photophysical radiationless transitions. In Principles of Molecular Photochemistry: An Introduction; Turro, N.J., Ramamurthy, V., Scaiano, J., Eds.; University Science Books: Sausalito, California, 2009; pp. 265–318. ISBN 978-1891389573. [Google Scholar]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef]
- Leung, N.L.C.; Xie, N.; Yuan, W.; Liu, Y.; Wu, Q.; Peng, Q.; Miao, Q.; Lam, J.W.Y.; Tang, B.Z. Restriction of intramolecular motions: The general mechanism behind aggregation-induced emission. Chem. Eur. J. 2014, 20, 15349–15353. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Zhuang, Z.; Zhao, Z.; Tang, B.Z. AIEgens based on main group heterocycles. J. Mater. Chem. C 2018, 6, 11835–11852. [Google Scholar] [CrossRef]
- He, Z.; Ke, C.; Tang, B.Z. Journey of aggregation-induced emission research. ACS Omega 2018, 3, 3267–3277. [Google Scholar] [CrossRef]
- Yamada, S.; Mitsuda, A.; Miyano, K.; Tanaka, T.; Morita, M.; Agou, T.; Kubota, T.; Konno, T. Development of novel solid-state light-emitting materials based on pentafluorinated tolane fluorophores. ACS Omega 2018, 3, 9105–9113. [Google Scholar] [CrossRef]
- Yamada, S.; Morita, M.; Agou, T.; Kubota, T.; Ichikawa, T.; Konno, T. Thermoresponsive luminescence properties of polyfluorinated bistolane-type light-emitting liquid crystals. Org. Biomol. Chem. 2018, 16, 5609–5617. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Miyano, K.; Konno, T.; Agou, T.; Kubota, T.; Hosokai, T. Fluorine-containing bistolanes as light-emitting liquid crystalline molecules. Org. Biomol. Chem. 2017, 15, 5949–5958. [Google Scholar] [CrossRef]
- Yamada, S.; Morita, M.; Konno, T. Multi-color photoluminescence induced by electron-density distribution of fluorinated bistolane derivatives. J. Fluorine Chem. 2017, 202, 54–64. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, D.; Zhang, M.; Cui, M.; Li, Q.; Fan, Y.; Wang, H.; Zeng, Z. N-fused ring strategy toward orange/yellow light-emitting liquid crystalline molecules. Dyes Pig. 2018, 159, 115–120. [Google Scholar] [CrossRef]
- Yamada, S.; Rokusha, Y.; Kawano, R.; Fujisawa, K.; Tsutsumi, O. Mesogenic gold complexes showing aggregation-induced enhancement of phosphorescence in both crystalline and liquid-crystalline phases. Faraday Discuss. 2017, 196, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Billa, M.R.; Kitney, S.P.; Kelly, S.M. Symmetrical carbazole-fluorene-carbazole nematic liquid crystals as electroluminescent organic semiconductors. Liq. Cryst. 2017, 45, 965–979. [Google Scholar] [CrossRef]
- Achalkumar, A.S.; Veerabhadraswamy, B.N.; Hiremath, U.S.; Rao, D.S.S.; Prasad, S.K.; Yelamaggad, C.V. Photoluminescent discotic liquid crystals derived from tris(N-salicylideneaniline) and stilbene conjugates: Structure-property correlations. Dyes Pig. 2016, 132, 291–305. [Google Scholar] [CrossRef]
- Yamada, S.; Tanaka, T.; Ichikawa, T.; Konno, T. Novel V- and Y-shaped light-emitting liquid crystals with pentafluorinated bistolane-based luminophores. ACS Omega 2019, 4, 3922–3932. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Frish, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Peterson, G.A.; et al. Gaussian 09; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Okuno, K.; Shigeta, Y.; Kishi, R.; Nakano, M. Non-empirical tuning of CAM-B3LYP functional in time-dependent density functional theory for excitation energies of diarylethene derivatives. Chem. Phys. Lett. 2013, 585, 201–206. [Google Scholar] [CrossRef]
- Andzelm, J.; Kölmel, C.; Klamt, A. Incorporation of solvent effects into density functional calculations of molecular energies and geometries. J. Chem. Phys. 1995, 103, 9312–9320. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.R.; Meek, T.L.; Allred, A.L.; Allen, L.C. Evaluation and test of Pauling’s electronegativity scale. J. Phys. Chem. A 2000, 104, 5867–5871. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Flack, H.D.; Bernardinelli, G. Reporting and evaluating absolute-structure and absolute-configuration determinations. J. Appl. Cryst. 2000, 33, 1143–1148. [Google Scholar] [CrossRef]
- Coates, D.; Gray, G.W. Optical studies of the amorphous liquid-cholesteric liquid crystal transition: The “blue phase”. Phys. Lett. A 1973, 45, 115–116. [Google Scholar] [CrossRef]
- Meiboom, S.; Sethna, J.P.; Anderson, P.W.; Brinkman, W.F. Theory of the blue phase of cholesteric liquid crystals. Phys. Rev. Lett. 1981, 46, 1216–1219. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yokota, M.; Hisakado, Y.; Yang, H.; Kajiyama, T. Polymer-stabilized liquid crystal blue phases. Nat. Mater. 2002, 1, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, A.; Sato, M.; Rokunohe, J. A blue phase observed for a novel chiral compound possessing molecular biaxiality. J. Mater. Chem. 2005, 15, 3285–3290. [Google Scholar] [CrossRef]
- Coles, H.J.; Pivnenko, M.N. Liquid crystal ‘blue phases’ with a wide temperature range. Nature 2005, 436, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Takahashi, H.; Ushio, T. New enantiomeric resolution phenomenon of racemic crystals: Preferential enrichment. J. Synth. Org. Chem. Jpn. 1998, 56, 22–33. [Google Scholar] [CrossRef]
- Cao, W.; Munoz, A.; Palffy-Muhoray, P.; Taheri, B. Lasing in a three-dimentional photonic crystal of the liquid crystal blue phase II. Nat. Mater. 2002, 1, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Mashiko, S.; Kikuchi, H.; Uchida, K.; Nagamura, T. Laser emission from a polymer-stabilized liquid-crystalline blue phase. Adv. Mater. 2006, 18, 48–51. [Google Scholar] [CrossRef]
- Yoshizawa, A. Material design for blue phase liquid crystals and their electro-optical effects. RSC Adv. 2013, 3, 25475–25497. [Google Scholar] [CrossRef]









| Compound | Phase Sequence and Temperature (Enthalpy, kJ mol−1) 1,2 | |
|---|---|---|
| S-2a | Heating | Cr1 60 (−16.6) Cr2 130 (58.1) N* 148 (0.35) Iso |
| Cooling | Iso 148 (–0.44) BP3 146 (–)4 N* 112 (−0.84) SmA 97 (−29.2) Cr1 | |
| R-2a | Heating | Cr1 60 (−29.6) Cr2 130 (50.9) N* 148 (0.35) Iso |
| Cooling | Iso 148 (−0.31) BP3 147 (–)4 N* 111 (−0.62) SmA 102 (−29.0) Cr1 | |
| rac-2a | Heating | Cr1 83 (−12.7) Cr2 118 (43.1) SmA 126 (−)3 N 155 (0.33) Iso |
| Cooling | Iso 154 (−0.33) N 115 (−0.44) SmA 102 (−25.2) Cr1 | |
| S-2b | Heating | Cr1 74 (−16.4) Cr2 130 (59.2) N* 143 (0.38) Iso |
| Cooling | Iso 144 (−0.52) BP3 142 (–)4 N* 116 (−0.84) SmA 89 (−28.6) Cr1 | |
| rac-2b | Heating | Cr1 75 (−12.1) Cr2 110 (−3.2) Cr3 130 (43.2) N 142 (0.28) Iso |
| Cooling | Iso 143 (−0.38) N 115 (−0.65) SmA 88 (−19.8) Cr2 85 (−0.24) Cr1 | |
| Compound | λabs (nm) (ε × 10−3 L mol−1 cm−1) 1 | λPL (nm) 2 | ΦPL 3 |
|---|---|---|---|
| S-2a | 330 (54.3), 348 (shoulder, 40.3) | 401 | 0.80 |
| R-2a | 330 (71.2), 348 (shoulder, 52.9) | 401 | 0.81 |
| rac-2a | 330 (54.3), 348 (shoulder, 40.3) | 401 | 0.80 |
| S-2b | 331 (61.3), 349 (shoulder, 45.6) | 401 | 0.88 |
| rac-2b | 331 (60.3), 349 (shoulder, 44.7) | 401 | 0.76 |
| Compound | λPL (nm) 1 | ΦPL 2 |
|---|---|---|
| S-2a | 445 | 0.38 |
| R-2a | 438 | 0.48 |
| rac-2a | 440 | 0.42 |
| S-2b | 453 | 0.54 |
| rac-2b | 441 | 0.55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Miyano, K.; Agou, T.; Kubota, T.; Konno, T. 2-Chloroalkoxy-Substituted Pentafluorinated Bistolanes as Novel Light-Emitting Liquid Crystals. Crystals 2019, 9, 195. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040195
Yamada S, Miyano K, Agou T, Kubota T, Konno T. 2-Chloroalkoxy-Substituted Pentafluorinated Bistolanes as Novel Light-Emitting Liquid Crystals. Crystals. 2019; 9(4):195. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040195
Chicago/Turabian StyleYamada, Shigeyuki, Kazuya Miyano, Tomohiro Agou, Toshio Kubota, and Tsutomu Konno. 2019. "2-Chloroalkoxy-Substituted Pentafluorinated Bistolanes as Novel Light-Emitting Liquid Crystals" Crystals 9, no. 4: 195. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040195