Assembly of Imidazolyl-Substituted Nitronyl Nitroxides into Ferromagnetically Coupled Chains
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Synthetic Procedures
2.2.1. 4,4,5,5-Tetramethyl-2-(4,5-dimethyl-1H-imidazolyl)-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl (1a)
2.2.2. 1-Tosyloxy-1-oxo-1H-1λ5-benzo[d][1,2]iodoxol-3-one (IBX-OTs)
2.2.3. 4,5-Dichloro-1H-imidazole-2-carbaldehyde
2.2.4. 4,4,5,5-Tetramethyl-2-(4,5-dichloro-1H-imidazolyl)-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl (1b)
2.3. Single-Crystal X-Ray Diffraction Analysis
2.4. Magnetic Measurements
2.5. Computational Details
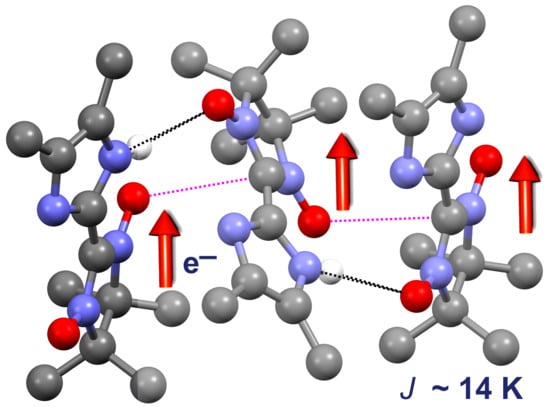
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veciana, J.; Iwamura, H. Organic magnets. MRS Bull. 2000, 25, 41–51. [Google Scholar] [CrossRef]
- Novoa, J.J.; Deumal, M. The mechanism of the through-space magnetic interactions in purely organic molecular magnets. In π-Electron Magnetism; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2001; Volume 100, pp. 34–60. [Google Scholar] [CrossRef]
- Hernàndez, E.; Mas, M.; Molins, E.; Rovira, C.; Veciana, J. Hydrogen bonds as a crystal design element for organic molecular solids with intermolecular ferromagnetic interactions. Angew. Chem. Int. Ed. 1993, 32, 882–884. [Google Scholar] [CrossRef]
- Cirujeda, J.; Hernàndez-Gasio, E.; Rovira, C.; Stanger, J.-L.; Turek, P.; Veciana, J. Role of hydrogen bonds in the propagation of ferromagnetic interactions in organic molecular solids. Part 1—The p-hydroxyphenyl α-nitronyl aminoxyl radical case. J. Mater. Chem. 1995, 5, 243–252. [Google Scholar] [CrossRef]
- Cirujeda, J.; Ochando, L.E.; Amigó, J.M.; Rovira, C.; Rius, J.; Veciana, J. Structure determination from powder X-ray diffraction data of a hydrogen-bonded molecular solid with competing ferromagnetic and antiferromagnetic interactions: The 2-(3,4-Dihydroxyphenyl)-α-nitronyl nitroxide radical. Angew. Chem. Int. Ed. 1995, 34, 55–57. [Google Scholar] [CrossRef]
- Veciana, J.; Cirujeda, J.; Rovira, C.; Molins, E.; Novoa, J. Organic ferromagnets. Hydrogen bonded supramolecular magnetic organizations derived from hydroxylated phenyl α-nitronyl nitroxide radicals. J. Phys. 1996, 6, 1967–1986. [Google Scholar] [CrossRef]
- Naoki, Y.; Munetoshi, I.; Yuichiro, M.; Takanari, K.; Hidenari, I.; Shigeru, O. Unusually Large Magnetic Interactions Observed in Hydrogen-Bonded Nitronyl Nitroxides. Chem. Lett. 1997, 26, 251–252. [Google Scholar] [CrossRef]
- Poderoso, J.L.; González-Cabello, A.; Jürgens, O.; Vidal-Gancedo, J.; Veciana, J.; Torres, T.; Vázquez, P. Synthesis and magnetic coupling of a bis(nitronyl nitroxide radical derived from 1,2,4-triazole. Synth. Met. 2001, 121, 1830–1831. [Google Scholar] [CrossRef]
- Lang, A.; Pei, Y.; Ouahab, L.; Kahn, O. Synthesis, crystal structure, and magnetic properties of 5-methyl-1,2,4-triazole-nitronyl nitroxide: A one-dimensional compound with unusually large ferromagnetic intermolecular interactions. Adv. Mater. 1996, 8, 60–62. [Google Scholar] [CrossRef]
- Nagashima, H.; Hashimoto, N.; Inoue, H.; Yoshioka, N. Coexistence of an antiferromagnetically coupled dimer and isolated paramagnetic spin in 4-azaindol-2-yl nitronyl nitroxide crystal. New J. Chem. 2003, 27, 805–810. [Google Scholar] [CrossRef]
- Nagashima, H.; Fujita, S.; Inoue, H.; Yoshioka, N. Metamagnetic behavior observed in purely organic 5-azaindol-2-yl nitronyl nitroxide brick-wall architecture. Cryst. Growth Des. 2004, 4, 19–21. [Google Scholar] [CrossRef]
- Romanov, V.E.; Bagryanskaya, I.Y.; Gorbunov, D.E.; Gritsan, N.P.; Zaytseva, E.V.; Luneau, D.; Tretyakov, E.V. A crystallographic study of a novel tetrazolyl-substituted nitronyl nitroxide radical. Crystals 2018, 8, 334. [Google Scholar] [CrossRef]
- Amini, M.; Golabchifar, A.A.; Dehpour, A.R.; Pirali, H.M.; Shafiee, A. Synthesis and calcium channel antagonist activity of new 1,4-dihydropyridine derivatives containing dichloroimidazolyl substituents. Arzneimittelforschung 2002, 52, 21–26. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Fokin, S.V.; Rey, P. A Thorough investigation of the synthetic problems of vic-bis-hydroxylamine—The precursor of Ullman’s nitroxides. Mol. Cryst. Liq. Cryst. 1999, 334, 109–119. [Google Scholar] [CrossRef]
- Yusubov, M.S.; Svitich, D.Y.; Yoshimura, A.; Nemykin, V.N.; Zhdankin, V.V. 2-Iodoxybenzoic acid organosulfonates: Preparation, X-ray structure and reactivity of new, powerful hypervalent iodine(V) oxidants. Chem. Commun. 2013, 49, 11269–11271. [Google Scholar] [CrossRef] [PubMed]
- Yusubov, M.S.; Postnikov, P.S.; Yusubova, R.Y.; Yoshimura, A.; Jürjens, G.; Kirschning, A.; Zhdankine, V.V. 2-Iodoxybenzoic acid tosylates: The alternative to Dess–Martin periodinane oxidizing reagents. Adv. Synth. Catal. 2017, 359, 3207–3216. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX-97, Programs for Crystal Structure Analysis (Release 97-2); University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- SADABS, v. 2008-1; Bruker AXS: Madison, WI, USA, 2008.
- Spek, A.L. PLATON, a Multipurpose Crystallographic Tool (Version 10M); Utrecht University: Utrecht, The Netherlands, 2003. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Stree, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Pascal, P. Recherches magnetochimiques (premier memoire). Ann. Chim. Phys. 1910, 19, 5–70. [Google Scholar]
- Soda, T.; Kitagawa, Y.; Onishi, T.; Takano, Y.; Shigeta, Y.; Nagao, H.; Yoshioka, Y.; Yamaguchi, K. Ab Initio computations of effective exchange integrals for H–H, H–He–H and Mn2O2 complex: Comparison of broken-symmetry approaches. Chem. Phys. Lett. 2000, 319, 223–230. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wires Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Ullman, E.F.; Osiecki, J.H.; Boocok, D.G.B.; Darcy, R. Studies of stable free radicals. X. Nitronyl nitroxide monoradicals and biradicals as possible small molecule spin labels. J. Am. Chem. Soc. 1972, 94, 7049–7059. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Ovcharenko, V.I. The chemistry of nitroxide radicals in the molecular design of magnets. Russ. Chem. Rev. 2009, 78, 971–1012. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular nonbonded contact distances in organic crystal structures: Comparison with distances expected from van der Waals Radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Swank, D.D.; Landee, C.P.; Willett, R.D. Crystal structure and magnetic susceptibility of copper (II) chloride tetramethylsulfoxide [CuCl2(TMSO)] and copper (II) chloride monodimethylsulfoxide [CuCl2(DMSO)]: Ferromagnetic spin-½ Heisenberg linear chains. Phys. Rev. B 1979, 20, 2154–2162. [Google Scholar] [CrossRef]
- Baker, G.A.; Rushbrooke, G.S., Jr.; Gilbert, H.E. High-Temperature Series Expansions for the Spin-½ Heisenberg Model by the Method of Irreducible Representations of the Symmetric Group. Phys. Rev. 1964, 135, A1272–A1277. [Google Scholar] [CrossRef]
- Clarke, C.S.; Jornet-Somoza, J.; Mota, F.; Novoa, J.J.; Deumal, M. Origin of the Magnetic Bistability in Molecule-Based Magnets: A First-Principles Bottom-Up Study of the TTTA Crystal. J. Am. Chem. Soc. 2010, 132, 17817–17830. [Google Scholar] [CrossRef]
- Tolstikov, S.E.; Tretyakov, E.V.; Gorbunov, D.E.; Zhurko, I.F.; Fedin, M.V.; Romanenko, G.V.; Bogomyakov, A.S.; Gritsan, N.P.; Mazhukin, D.G. Reaction of Paramagnetic Synthon, Lithiated 4,4,5,5-Tetramethyl-4,5-dihydro-1H-imidazol-1-oxyl 3-oxide, with Cyclic Aldonitrones of the Imidazole Series. Chem. Eur. J. 2016, 22, 14598–14604. [Google Scholar] [CrossRef] [PubMed]









| Compound | 1a | 1b |
|---|---|---|
| Empirical formula | C12H19N4O2 | C10H13N4O2Cl2 |
| Formula weight | 251.31 | 292.14 |
| Temperature, K | 296(2) | 296(2) |
| Wavelength, Å | 0.71073 | 0.71073 |
| Crystal system | Orthorhombic | Orthorhombic |
| Space group | Pbca | Pbca |
| Unit cell dimensions a, Å | 8.7454(4) | 8.7195(5) |
| b, Å | 15.4621(8) | 15.460(1) |
| c, Å | 19.852(1) | 19.551(1) |
| Volume, Å3; Z | 2684.4(2); 8 | 2635.5(3); 8 |
| Density (calcd.), mg·m−3 | 1.244 | 1.473 |
| Abs. coefficient, mm−1 | 0.087 | 0.493 |
| F(000) | 1080 | 1208 |
| Crystal size, mm3 | 0.06 × 0.06 × 0.9 | 0.02 × 0.06 × 0.40 |
| θ range for data collection, ° | 4.0–25.0 | 2.6–26.0 |
| Index ranges | −10 ≤ h ≤ 10, −18 ≤ k ≤ 18, −23 ≤ l ≤ 23 | −10 ≤ h ≤10, −19 ≤ k ≤19, −24 ≤ l ≤24 |
| Reflections collected | 34103 | 29738 |
| Independent reflections | 2365 R(int) = 0.063 | 2603 R(int) = 0.104 |
| Completeness to θ, % | 99.5 | 99.8 |
| Data / restraints / parameters | 2365/0/169 | 2603/0/167 |
| Goodness-of-fit on F2 | 1.01 | 1.00 |
| Final R indices I > 2σ(I) | R1 = 0.0481, wR2 = 0.1338 | R1 = 0.0448, wR2 = 0.0863 |
| Final R indices (all data) | R1 = 0.0584, wR2 = 0.1461 | R1 = 0.0978, wR2 = 0.1049 |
| Largest diff. peak / hole, e⋅Å−3 | 0.22/−0.25 | 0.24/−0.22 |
| Compound | 1a | 1b | 1c |
|---|---|---|---|
| R(O2…O1), Å | 3.292(2) | 3.125(3) | 3.484(6) |
| R(O2…C3), Å | 3.145(2) | 3.050(3) | 3.702(7) |
| ∠N1–O2…O1′–N4′ | 118.3(2) | 121.6(2) | 180.0(5) |
| Jcalc, cm–1 | 14.6 | 16.5 | −36.7 |
| Jexp, cm−1 | 5.0 ± 0.3 a 4.7 ± 0.2 c | b − | −61.5 − |
| J′calc,d cm−1 | −0.31, 0.06 | −0.27, 0.01 | −0.9, −0.06 |
| θ′exp | −3.0 ± 0.2 | b | − |
| zJ′, K | −0.55 ± 0.02 | − | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanov, V.; Bagryanskaya, I.; Gritsan, N.; Gorbunov, D.; Vlasenko, Y.; Yusubov, M.; Zaytseva, E.; Luneau, D.; Tretyakov, E. Assembly of Imidazolyl-Substituted Nitronyl Nitroxides into Ferromagnetically Coupled Chains. Crystals 2019, 9, 219. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040219
Romanov V, Bagryanskaya I, Gritsan N, Gorbunov D, Vlasenko Y, Yusubov M, Zaytseva E, Luneau D, Tretyakov E. Assembly of Imidazolyl-Substituted Nitronyl Nitroxides into Ferromagnetically Coupled Chains. Crystals. 2019; 9(4):219. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040219
Chicago/Turabian StyleRomanov, Vasily, Irina Bagryanskaya, Nina Gritsan, Dmitry Gorbunov, Yulia Vlasenko, Mehman Yusubov, Elena Zaytseva, Dominique Luneau, and Evgeny Tretyakov. 2019. "Assembly of Imidazolyl-Substituted Nitronyl Nitroxides into Ferromagnetically Coupled Chains" Crystals 9, no. 4: 219. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9040219