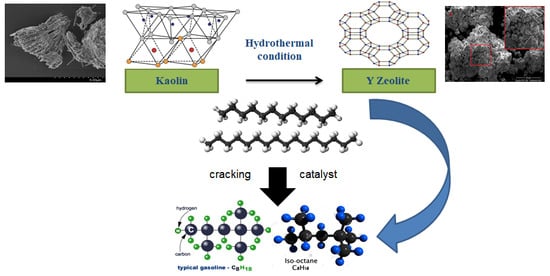
Synthesis and Characterization of Crystalline NaY-Zeolite from Belitung Kaolin as Catalyst for n-Hexadecane Cracking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Pre-Treatment of Natural Kaolin
2.3. Synthesis of NaY Zeolite
2.4. Characterization
2.5. Catalytic Cracking Test
3. Results
3.1. Characterization of Raw Materials
3.2. Synthesis of NaY Zeolite Seeds
3.3. Synthesis NaY Zeolite
3.4. Preparation of HY Zeolites as Cracking Catalysts
3.5. n-Hexadecane Catalytic Cracking
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dionisiou, N.S.; Matsi, T. Natural and Surfactant-Modified Zeolite for the Removal of Pollutants (Mainly Inorganic) from Natural Waters and Wastewaters; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Panias, D.; Xenidis, A.; Krestou, A. Materials and Processes for Uranium Removal from Contaminated Water; Elsevier Masson SAS: Paris, France, 2007; Volume 7. [Google Scholar]
- Swenson, P.; Tanchuk, B.; Bastida, E.; An, W.; Kuznicki, S.M. Water desalination and de-oiling with natural zeolite membranes—Potential application for purification of SAGD process water. Desalination 2012, 286, 442–446. [Google Scholar] [CrossRef]
- Coal, R.; Bay, R.; Adriatic, N. Use of Bacteria and Synthetic Zeolites in Remediation of Soil and Water Polluted with Superhigh-Organic-Sulfur. Water 2019, 11, 1419. [Google Scholar]
- Calabrese, L.; Proverbio, E. A Brief Overview on the Anticorrosion Performances of Sol-Gel Zeolite Coatings. Coatings 2019, 9, 409. [Google Scholar] [CrossRef]
- Davis, M. Ordered Porous Materials for Emerging Applications. Nature 2015, 10, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Primo, A.; Garcia, H. Zeolites as catalysts in oil refining. Chem. Soc. Rev. 2014, 43, 7548–7561. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.B.G.; Arshad, S.E. Hydrothermally synthesized zeolites based on kaolinite: A review. Appl. Clay Sci. 2014, 97–98, 215–221. [Google Scholar] [CrossRef]
- Lutz, W. Zeolite Y: Synthesis, Modification, and Properties—A Case Revisited. Adv. Mater. Sci. Eng. 2014, 2014, 724248. [Google Scholar] [CrossRef]
- Meng, X.; Xiao, F. Green Routes for Synthesis of Zeolites. Chem. Rev. 2014, 114, 1521–1543. [Google Scholar] [CrossRef]
- Adamczyk, Z. Hydrothermal Synthesis of Zeolites from Polish Coal Fly Ash. Pol. J. Environ. Stud. 2005, 14, 713–719. [Google Scholar]
- Margenot, A.J.; Parikh, S.J. IR Spectroscopy in Soil Analysis Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 913–928. [Google Scholar]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Sadjadi, S.; Lazzara, G.; Malmir, M.; Heravi, M.M. Pd nanoparticles immobilized on the poly-dopamine decorated halloysite nanotubes hybridized with N-doped porous carbon monolayer: A versatile catalyst for promoting Pd catalyzed reactions. J. Catal. 2018, 366, 245–257. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Notoa, R.; Riela, S. Halloysite nanotubes as support for metal-based catalysts. J. Mater. Chem. 2017, 5, 13276–13293. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Parmanti, I.Y.; Yunarti, R.T.; Sihombing, R.; Saragi, I.R. Synthesis and Characterization of Zeolite NaY from kaolin Bangka Belitung with variation of synthesis composition and crystallization time. J. Phys. Conf. Ser. 2018, 1095, 012043. [Google Scholar] [CrossRef] [Green Version]
- Melaningtyas, G.S.A.; Krisnandi, Y.K.; Ekananda, R. Synthesis and characterization of NaY zeolite from Bayat natural zeolite: Effect of pH on synthesis. IOP Conf. Ser. Mater. Sci. Eng. 2019, 496, 012042. [Google Scholar] [CrossRef]
- Zahara, Z.; Krisnandi, Y.K.; Wibowo, W.; Nurani, D.A.; Rahayu, D.U.C.; Haerudin, H. Synthesis and characterization of hierarchical ZSM-5 zeolite using various templates as cracking catalysts. AIP Conf. Proc. 2018, 2023, 020088. [Google Scholar]
- Saragi, I.R.; Krisnandi, Y.K.; Sihombing, R. Synthesis and Characterization HY Zeolite from Natural Aluminosilicate for n-Hexadecane Cracking. Mater. Today Proc. 2019, 13, 76–81. [Google Scholar] [CrossRef]
- Ming, D.W.; Dixon, J.B. Technique for the Separation of Clinoptilolite from Soils. Clays Clay Miner. 1987, 35, 4–7. [Google Scholar] [CrossRef]
- Gougazeh, M.; Buhl, J.C. Synthesis and characterization of zeolite A by hydrothermal transformation of natural Jordanian kaolin. J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 15, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Shi, C.; Wu, D.; He, S.; Ren, B. A Simple Method of Preparation of High Silica Zeolite y and Its Performance in the Catalytic Cracking of Cumene. J. Nanotechnol. 2016, 2016, 1486107. [Google Scholar] [CrossRef]
- Ginter, D.M.; Bell, A.T.; Radke, C.J. The effects of gel aging on the synthesis of NaY zeolite from colloidal silica. Zeolites 1992, 12, 742–749. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; de Menezes, S.M.C.; Goodfellow, R.; Granger, P. NMR Nomenclature: Nuclear Spin Properties and Conventions for Chemical Shifts. Solid State Nucl. Magn. Reson. 2002, 22, 458–483. [Google Scholar] [CrossRef]
- Feng, H.; Li, C.; Shan, H. Effect of Calcination Temperature of Kaolin Microspheres on the In situ Synthesis of ZSM-5. Catal. Lett. 2009, 129, 71–78. [Google Scholar] [CrossRef]
- Stylianou, M.; Inglezakis, V.; Agapiou, A.; Itskos, G.; Jetybayeva, A.; Loizidou, M. A comparative study on phyllosilicate and tectosillicate mineral structural properties. Desalin. Water Treat. 2018, 112, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Rohayati, Y.; Krisnandi, K.; Sihombing, R. Synthesis of ZSM-5 zeolite using Bayat natural zeolite as silica and alumina source. AIP Conf. Proc. 2017, 1862, 1–5. [Google Scholar]
- Abdullahi, T.; Harun, Z.; Othman, M.H.D. A review on sustainable synthesis of zeolite from kaolinite resources via hydrothermal process. Adv. Powder Technol. 2017, 28, 1827–1840. [Google Scholar] [CrossRef]
- Stepanov, A.G. Basics of Solid-State NMR for Application in Zeolite Science; Elsevier B.V.: Amsterdam, The Netherlands, May 2016. [Google Scholar]
- Li, S.; Deng, F. Recent Advances of Solid-State NMR Studies on Zeolites, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 78. [Google Scholar]
- Abbas, A.S.; Abbas, R.N. Preparation and Characterization of Nay Zeolite for Biodiesel Production. Iraqi J. Chem. Pet. Eng. 2015, 16, 19–29. [Google Scholar]
- Foster, M.D.; Rivin, I.; Treacy, M.M.J.; Friedrichs, O.D. A geometric solution to the largest-free-sphere problem in zeolite frameworks. Microporous Mesoporous Mater. 2006, 90, 32–38. [Google Scholar] [CrossRef]
- Gore, K.U.; Abraham, A.; Hegde, S.G.; Kumar, R.; Amoureux, J.; Ganapathy, S. 29SiMAS and 27Al/3Q-MAS NMR Studies of High Silica USY Zeolites. J. Phys. Chem. B 2002, 106, 6115–6120. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]















| Chemical Composition | %-Mass Kaolin | %-Mass Metakaolin | %-Mass Extracted Silica |
|---|---|---|---|
| Na2O/K2O | 4.48 | 5.31 | 1.353 |
| Al2O3 | 33.39 | 33.39 | 3.952 |
| SiO2 | 57.11 | 56.70 | 90.487 |
| Cl | 0.59 | 0.53 | 0.581 |
| CaO | 0.68 | 0.59 | 0.513 |
| Fe2O3 | 3.75 | 3.57 | 1.567 |
| SiO2/Al2O3 | 1.71 | 1.69 | 22.89 |
| Chemical Composition | NaY-LDX Mass (%) | NaY-MKSE Mass (%) | NaY-SE Mass (%) |
|---|---|---|---|
| Na2O | - | 2.70 | 1.77 |
| Al2O3 | 20.32 | 18.67 | 18.03 |
| SiO2 | 75.86 | 72.62 | 74.15 |
| P2O5 | 1.57 | 1.12 | 1.26 |
| Cl | 1.05 | 0.76 | 0.88 |
| CaO | 0.95 | 0.73 | 0.79 |
| Fe2O3 | 0.26 | 2.80 | 2.38 |
| SiO2/Al2O3 | 3.73 | 3.88 | 4.11 |
| Materials | Surface Area Peak * 2θ (6.1°; 15.6°; 22.1°) | Crystallinity |
|---|---|---|
| Commercial NaY | 376.88 | 45.00% |
| NaY-SW | 4.314 | 0.51% |
| NaY-LDX | 820.69 | 100.00% |
| NaY-MKSE | 149.41 | 18.22% |
| NaY-SE | 293.85 | 35.80% |
| Materials | Surface Area | Pore Volume (V) | rpore | ||||
|---|---|---|---|---|---|---|---|
| SBET (m2/g) | SEXT (m2/g) | Smikro (m2/g) | Vtotal (cm3/g) | Vmeso (cm3/g) | Vmikro (cm3/g) | (nm) | |
| NaY-LDX | 451.9 | 7.083 | 444.8 | 0.259 | 0.025 | 0.234 | 1.1468 |
| NaY-MKSE | 412.7 | 5.810 | 406.9 | 0.235 | 0.021 | 0.214 | 1.1414 |
| NaY-SE | 383.2 | 7.180 | 376.2 | 0.219 | 0.019 | 0.200 | 1.1463 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krisnandi, Y.K.; Saragi, I.R.; Sihombing, R.; Ekananda, R.; Sari, I.P.; Griffith, B.E.; Hanna, J.V. Synthesis and Characterization of Crystalline NaY-Zeolite from Belitung Kaolin as Catalyst for n-Hexadecane Cracking. Crystals 2019, 9, 404. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9080404
Krisnandi YK, Saragi IR, Sihombing R, Ekananda R, Sari IP, Griffith BE, Hanna JV. Synthesis and Characterization of Crystalline NaY-Zeolite from Belitung Kaolin as Catalyst for n-Hexadecane Cracking. Crystals. 2019; 9(8):404. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9080404
Chicago/Turabian StyleKrisnandi, Yuni K., Indah R. Saragi, Riwandi Sihombing, Rizki Ekananda, Indah P. Sari, Benjamin E. Griffith, and John V. Hanna. 2019. "Synthesis and Characterization of Crystalline NaY-Zeolite from Belitung Kaolin as Catalyst for n-Hexadecane Cracking" Crystals 9, no. 8: 404. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9080404