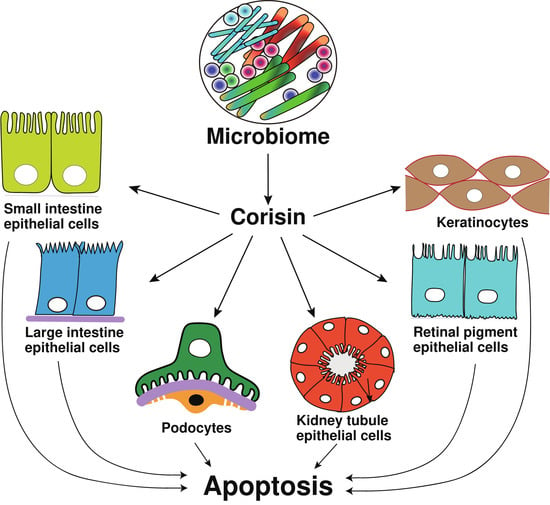
A Microbiome-Derived Peptide Induces Apoptosis of Cells from Different Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Evaluation of Cell Apoptosis
2.4. Flow Cytometry Analysis
2.5. Western Blotting
2.6. Evaluation of Reactive Oxygen Species during Corisin-Induced Apoptosis
2.7. Mitochondrial Membrane Potential Assay
2.8. Statistical Analysis
3. Results
3.1. Differential Proapoptotic Activity of Corisin and Corisin-like Peptide on Cells from Different Tissues
3.2. Corisin Potentiates the Proapoptotic Activity of TGFβ1 on Podocytes
3.3. Increased Generation of Reactive Oxygen Species and Loss of Mitochondrial Membrane Integrity during Apoptosis Induced by Corisin
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Gillies, L.A.; Kuwana, T. Apoptosis regulation at the mitochondrial outer membrane. J. Cell Biochem. 2014, 115, 632–640. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wang, H.; Huang, C.; Huang, Y.; Li, J. Endoplasmic reticulum stress is the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in liver fibrosis. Inflamm. Res. 2015, 64, 1–7. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Strasser, A.; Whittingham, S.; Vaux, D.L.; Bath, M.L.; Adams, J.M.; Cory, S.; Harris, A.W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl. Acad. Sci. USA 1991, 88, 8661–8665. [Google Scholar] [CrossRef] [Green Version]
- Sly, L.M.; Hingley-Wilson, S.M.; Reiner, N.E.; McMaster, W.R. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J. Immunol. 2003, 170, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Rohn, T.T. The role of caspases in Alzheimer’s disease; potential novel therapeutic opportunities. Apoptosis 2010, 15, 1403–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, N.A.; Fisher, J.K.; Austgen, K.; VandenBerg, S.; Huang, E.J.; Oakes, S.A. Blocking the mitochondrial apoptotic pathway preserves motor neuron viability and function in a mouse model of amyotrophic lateral sclerosis. J. Clin. Investig. 2010, 120, 3673–3679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blesa, J.; Przedborski, S. Parkinson’s disease: Animal models and dopaminergic cell vulnerability. Front. Neuroanat. 2014, 8, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesch, G.H.; Ma, F.Y.; Nikolic-Paterson, D.J. Targeting apoptosis signal-regulating kinase 1 in acute and chronic kidney disease. Anat. Rec. 2020, 303, 2553–2560. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Horowitz, J.C. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc. Am. Thorac. Soc. 2006, 3, 350–356. [Google Scholar] [CrossRef]
- Adamiec-Mroczek, J.; Zajac-Pytrus, H.; Misiuk-Hojlo, M. Caspase-Dependent Apoptosis of Retinal Ganglion Cells during the Development of Diabetic Retinopathy. Adv. Clin. Exp. Med. 2015, 24, 531–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebane, A.; Zimmermann, M.; Aab, A.; Baurecht, H.; Koreck, A.; Karelson, M.; Abram, K.; Metsalu, T.; Pihlap, M.; Meyer, N.; et al. Mechanisms of IFN-gamma-induced apoptosis of human skin keratinocytes in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2012, 129, 1297–1306. [Google Scholar] [CrossRef]
- Teringova, E.; Tousek, P. Apoptosis in ischemic heart disease. J. Transl. Med. 2017, 15, 87. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Linton, M.F.; Babaev, V.R.; Huang, J.; Linton, E.F.; Tao, H.; Yancey, P.G. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circ. J. 2016, 80, 2259–2268. [Google Scholar] [CrossRef] [Green Version]
- Ebersole, J.L.; Kirakodu, S.S.; Gonzalez, O.A. Oral microbiome interactions with gingival gene expression patterns for apoptosis, autophagy and hypoxia pathways in progressing periodontitis. Immunology 2021, 162, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Kirakodu, S.S.; Neumann, E.; Orraca, L.; Gonzalez Martinez, J.; Gonzalez, O.A. Oral Microbiome and Gingival Tissue Apoptosis and Autophagy Transcriptomics. Front. Immunol. 2020, 11, 585414. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Yang, P.; Liu, X.; Lu, L.; Chen, Y.; Zhong, X.; Li, Z.; Liu, H.; Ou, C.; et al. Trimethylamine-N-Oxide Promotes Vascular Calcification Through Activation of NLRP3 (Nucleotide-Binding Domain, Leucine-Rich-Containing Family, Pyrin Domain-Containing-3) Inflammasome and NF-kappaB (Nuclear Factor kappaB) Signals. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 751–765. [Google Scholar] [CrossRef]
- Ohtani, N.; Kawada, N. Role of the gut-liver axis in liver inflammation, fibrosis, and cancer: A special focus on the gut microbiota relationship. Hepatol. Commun. 2019, 3, 456–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandro-Gabazza, C.N.; Kobayashi, T.; Yasuma, T.; Toda, M.; Kim, H.; Fujimoto, H.; Hataji, O.; Takeshita, A.; Nishihama, K.; Okano, T.; et al. A Staphylococcus pro-apoptotic peptide induces acute exacerbation of pulmonary fibrosis. Nat. Commun. 2020, 11, 1539. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococci in the human microbiome: The role of host and interbacterial interactions. Curr. Opin. Microbiol. 2020, 53, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S. Cellular Stress Responses and Gut Microbiota in Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2018, 2018, 7192646. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.; Chan, A.S.; Zhang, C.B.; Garcia Cordoba, C.A.; Zhang, Y.Y.; To, K.F.; Leung, K.T.; Lan, H.Y.; Tang, P.M. TGF-beta1 Signaling: Immune Dynamics of Chronic Kidney Diseases. Front. Med. 2021, 8, 628519. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiki, H.; Okano, Y.; Yasuma, T.; Toda, M.; Takeshita, A.; Abdel-Hamid, A.M.; Fridman D’Alessandro, V.; Tsuruga, T.; D’Alessandro-Gabazza, C.N.; Katayama, K.; et al. A Microbiome-Derived Peptide Induces Apoptosis of Cells from Different Tissues. Cells 2021, 10, 2885. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10112885
Saiki H, Okano Y, Yasuma T, Toda M, Takeshita A, Abdel-Hamid AM, Fridman D’Alessandro V, Tsuruga T, D’Alessandro-Gabazza CN, Katayama K, et al. A Microbiome-Derived Peptide Induces Apoptosis of Cells from Different Tissues. Cells. 2021; 10(11):2885. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10112885
Chicago/Turabian StyleSaiki, Haruko, Yuko Okano, Taro Yasuma, Masaaki Toda, Atsuro Takeshita, Ahmed M. Abdel-Hamid, Valeria Fridman D’Alessandro, Tatsuki Tsuruga, Corina N. D’Alessandro-Gabazza, Kan Katayama, and et al. 2021. "A Microbiome-Derived Peptide Induces Apoptosis of Cells from Different Tissues" Cells 10, no. 11: 2885. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10112885