BTLA Expression in CLL: Epigenetic Regulation and Impact on CLL B Cell Proliferation and Ability to IL-4 Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Peripheral Blood Mononuclear Cells (PBMCs) Isolation
2.3. The Study of the Epigenetic Regulation of BTLA Expression by miR-155-5p
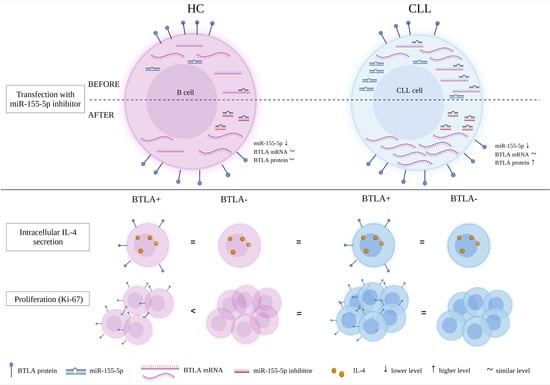
2.3.1. Cell Culture and Transfection
2.3.2. miR-155-5p Expression Determination
2.3.3. Determination of BTLA mRNA Expression
2.3.4. Assessment of BTLA Protein Expression
2.4. The Study on the Influence of BTLA Expression on Cell Function
Assessment of B Cell Capacity to IL-4 Secretion and Proliferation
2.5. Statistical Analysis
3. Results
3.1. The Study of the Epigenetic Regulation of BTLA Expression by miR-155-5p
3.1.1. In Silico Analysis of miR-155-5p and BTLA Interaction
3.1.2. The Study of the Epigenetic Regulation of BTLA Expression by miR-155-5p in CLL Patients
mRNA BTLA Is Overexpressed in CLL Patients
miR-155-5p Is Overexpressed in CLL Patients
The Effect of miR-155-5p Inhibition on BTLA Protein Expression on B Cells
3.1.3. The Study of the Epigenetic Regulation of BTLA Expression by miR-155-5p in MEC-1 Cell Line
BTLA mRNA and miR-155-5p Is Upregulated in MEC-1 Cells
Influence of miR-155-5p Inhibition on the BTLA Protein in MEC-1 Cells
3.2. The Study on the Influence of Abnormal BTLA Expression on B Cell Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallek, M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2019, 94, 1266–1287. [Google Scholar] [CrossRef] [Green Version]
- Arruga, F.; Gyau, B.B.; Iannello, A.; Vitale, N.; Vaisitti, T.; Deaglio, S. Immune Response Dysfunction in Chronic Lymphocytic Leukemia: Dissecting Molecular Mechanisms and Microenvironmental Conditions. Int. J. Mol. Sci. 2020, 21, 1825. [Google Scholar] [CrossRef] [Green Version]
- Frydecka, I.; Kosmaczewska, A.; Bocko, D.; Ciszak, L.; Wolowiec, D.; Kuliczkowski, K.; Kochanowska, I. Alterations of the expression of T-cell-related costimulatory CD28 and downregulatory CD152 (CTLA-4) molecules in patients with B-cell chronic lymphocytic leukaemia. Br. J. Cancer 2004, 90, 2042–2048. [Google Scholar] [CrossRef] [Green Version]
- Beyer, M.; Kochanek, M.; Darabi, K.; Popov, A.; Jensen, M.; Endl, E.; Knolle, P.A.; Thomas, R.K.; von Bergwelt-Baildon, M.; Debey, S.; et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood 2005, 106, 2018–2025. [Google Scholar] [CrossRef] [Green Version]
- Motta, M.; Rassenti, L.; Shelvin, B.J.; Lerner, S.; Kipps, T.J.; Keating, M.J.; Wierda, W.G. Increased expression of CD152 (CTLA-4) by normal T lymphocytes in untreated patients with B-cell chronic lymphocytic leukemia. Leukemia 2005, 19, 1788–1793. [Google Scholar] [CrossRef] [Green Version]
- Kosmaczewska, A.; Ciszak, L.; Suwalska, K.; Wolowiec, D.; Frydecka, I. CTLA-4 overexpression in CD19+/CD5+ cells correlates with the level of cell cycle regulators and disease progression in B-CLL patients. Leukemia 2005, 19, 301–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, A.D.; Hegde, G.V.; Dickinson, J.D.; Mittal, A.K.; Lynch, J.C.; Eudy, J.D.; Armitage, J.O.; Bierman, P.J.; Bociek, R.G.; Devetten, M.P.; et al. ATM, CTLA4, MNDA, and HEM1 in high versus low CD38 expressing B-cell chronic lymphocytic leukemia. Clin. Cancer Res. 2007, 13, 5295–5304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciszak, L.; Frydecka, I.; Wolowiec, D.; Szteblich, A.; Kosmaczewska, A. CTLA-4 affects expression of key cell cycle regulators of G0/G1 phase in neoplastic lymphocytes from patients with chronic lymphocytic leukaemia. Clin. Exp. Med. 2016, 16, 317–332. [Google Scholar] [CrossRef] [Green Version]
- Ciszak, L.; Frydecka, I.; Wolowiec, D.; Szteblich, A.; Kosmaczewska, A. Patients with chronic lymphocytic leukaemia (CLL) differ in the pattern of CTLA-4 expression on CLL cells: The possible implications for immunotherapy with CTLA-4 blocking antibody. Tumor Biol. 2016, 37, 4143–4157. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, N.; Gavrieli, M.; Sedy, J.R.; Yang, J.; Fallarino, F.; Loftin, S.K.; Hurchla, M.A.; Zimmerman, N.; Sim, J.; Zang, X.; et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 2003, 4, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Sedy, J.R.; Gavrieli, M.; Potter, K.G.; Hurchla, M.A.; Lindsley, R.C.; Hildner, K.; Scheu, S.; Pfeffer, K.; Ware, C.F.; Murphy, T.L.; et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 2005, 6, 90–98. [Google Scholar] [CrossRef]
- Murphy, T.L.; Murphy, K.M. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu. Rev. Immunol. 2010, 28, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Vendel, A.C.; Calemine-Fenaux, J.; Izrael-Tomasevic, A.; Chauhan, V.; Arnott, D.; Eaton, D.L. B and T lymphocyte attenuator regulates B cell receptor signaling by targeting Syk and BLNK. J. Immunol. 2009, 182, 1509–1517. [Google Scholar] [CrossRef]
- Thibult, M.L.; Rivals, J.P.; Mamessier, E.; Gertner-Dardenne, J.; Pastor, S.; Speiser, D.E.; Derré, L.; Olive, D. CpG-ODN-induced sustained expression of BTLA mediating selective inhibition of human B cells. J. Mol. Med. 2013, 91, 195–205. [Google Scholar] [CrossRef] [PubMed]
- M’Hidi, H.; Thibult, M.-L.; Chetaille, B.; Rey, F.; Bouadallah, R.; Nicollas, R. High expression of the inhibitory receptor BTLA in T-follicular helper cells and in B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Am. J. Clin. Pathol. 2009, 132, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.; Shankar, E.M.; Che, K.F.; Saeidi, A.; Ellegård, R.; Barathan, M.; Velu, V.; Kamarulzaman, A. Molecular signatures of T-cell inhibition in HIV-1 infection. Retrovirology 2013, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, N.; Nakajima, H. Coinhibitory Molecules in Autoimmune Diseases. Clin. Dev. Immunol. 2012, 2012, 269756. [Google Scholar] [CrossRef] [Green Version]
- Karabon, L.; Partyka, A.; Ciszak, L.; Pawlak-Adamska, E.; Tomkiewicz, A.; Bojarska-Junak, A.; Roliński, J.; Wołowiec, D.; Wrobel, T.; Frydecka, I.; et al. Abnormal Expression of BTLA and CTLA-4 Immune Checkpoint Molecules in Chronic Lymphocytic Leukemia Patients. J. Immunol. Res. 2020, 2020, 6545921. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved Seed Pairing, Often Flanked by Adenosines, Indicates that Thousands of Human Genes are MicroRNA Targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei, H.; Fathullahzadeh, S.; Khanmohammadi, R.; Darijani, M.; Momeni, F.; Masoudifar, A.; Goodarzi, M.; Mardanshah, O.; Stenvang, J.; Jaafari, M.R.; et al. State of the art in microRNA as diagnostic and therapeutic biomarkers in chronic lymphocytic leukemia. J. Cell Physiol. 2018, 233, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, Y.; Zhang, H. Identification of key miRNA-gene pairs in chronic lymphocytic leukemia through integrated analysis of mRNA and miRNA microarray. Oncol. Lett. 2018, 15, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Balatti, V.; Acunzo, M.; Pekarky, Y.; Croce, C.M. Novel Mechanisms of Regulation of miRNAs in CLL. Trends Cancer 2016, 2, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Katsaraki, K.; Karousi, P.; Artemaki, P.; Scorilas, A.; Pappa, V.; Kontos, C.K.; Papageorgiou, S.G. MicroRNAs: Tiny Regulators of Gene Expression with Pivotal Roles in Normal B-Cell Development and B-Cell Chronic Lymphocytic Leukemia. Cancers 2021, 13, 539. [Google Scholar] [CrossRef] [PubMed]
- Van, R.K.; Calin, G.A. MicroRNAs in chronic lymphocytic leukemia: miRacle or miRage for prognosis and targeted therapies? Semin. Oncol. 2016, 43, 209–214. [Google Scholar]
- Loeb, G.B.; Khan, A.A.; Canner, D.; Hiatt, J.B.; Shendure, J.; Darnell, R.B.; Leslie, C.S.; Rudensky, A.Y. Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Mol. Cell 2012, 48, 760–770. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Nie, W.; Jin, Y.; Zhuo, A.; Zang, Y.; Xiu, Q. B and T Lymphocyte Attenuator is a Target of miR-155 during Naive CD4+ T Cell Activation. Iran. J. Immunol. 2016, 13, 89–99. [Google Scholar]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.J.; Montserrat, E.; Rai, K.R.; et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008, 111, 5446–5456. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, M.; Ratho, R.K.; Chawla, Y.; Singh, M.P. Evaluating the role of low-speed centrifugation towards transfecting human peripheral blood mononuclear cell culture. Indian J. Med. Microbiol. 2014, 32, 164–168. [Google Scholar] [CrossRef]
- Karabon, L.; Partyka, A.; Jasek, M.; Lech-Maranda, E.; Grzybowska-Izydorczyk, O.; Bojarska-Junak, A.; Pawlak-Adamska, E.; Tomkiewicz, A.; Robak, T.; Rolinski, J.; et al. Intragenic Variations in BTLA Gene Influence mRNA Expression of BTLA Gene in Chronic Lymphocytic Leukemia Patients and Confer Susceptibility to Chronic Lymphocytic Leukemia. Arch. Immunol. Ther. Exp. 2016, 64, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mocellin, S.; Benna, C.; Pilati, P. Coinhibitory molecules in cancer biology and therapy. Cytokine Growth Factor Rev. 2013, 24, 147–161. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Gonzalez-Rodriguez, A.P.; Payer, R.; González-García, E.; López-Soto, A.; Gonzalez, S. BTLA/HVEM Axis Induces NK Cell Immunosuppression and Poor Outcome in Chronic Lymphocytic Leukemia. Cancers 2021, 13, 1766. [Google Scholar] [CrossRef] [PubMed]
- Siepel, A.; Bejerano, G.; Pedersen, J.S.; Hinrichs, A.S.; Hou, M.; Rosenbloom, K.; Clawson, H.; Spieth, J.; Hillier, L.W.; Richards, S.; et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005, 15, 1034–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, L.W.; Fletcher, S.; Wilton, S. Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cell. Mol. Life Sci. 2012, 69, 3613–3634. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E. Nonlinear partial differential equations and applications: Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef] [Green Version]
- Calin, G.A.; Cimmino, A.; Fabbri, M.; Ferracin, M.; Wojcik, S.E.; Shimizu, M.; Taccioli, C.; Zanesi, N.; Garzon, R.; Aqeilan, R.I.; et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc. Natl. Acad. Sci. USA 2008, 105, 5166–5171. [Google Scholar] [CrossRef] [Green Version]
- Jurkovicova, D.; Magyerkova, M.; Kulcsar, L.; Krivjanska, M.; Krivjansky, V.; Gibadulinova, A. miR-155 as a diagnostic and prognostic marker in hematological and solid malignancies. Neoplasma 2014, 61, 241–251. [Google Scholar] [CrossRef]
- Eis, P.; Tam, W.; Sun, L.; Chadburn, A.; Li, Z.; Gomez, M.F.; Lund, E.; Dahlberg, J.D. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc. Natl. Acad. Sci. USA 2005, 102, 3627–3632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kluiver, J.; Poppema, S.; De Jong, D.; Blokzijl, T.; Harms, G.; Jacobs, S.; Kroesen, B.J.; van den Berg, A. BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J. Pathol. 2005, 207, 243–249. [Google Scholar] [CrossRef]
- Marton, S.; Garcia, M.R.; Robello, C.; Persson, H.; Trajtenberg, F.; Pritsch, O.; Rovira, C.; Naya, H.; Dighiero, G.; Cayota, A. Small RNAs analysis in CLL reveals a deregulation of miRNA expression and novel miRNA candidates of putative relevance in CLL pathogenesis. Leukemia 2008, 22, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Filip, A.A.; Grenda, A.; Popek, S.; Koczkodaj, D.; Michalak-Wojnowska, M.; Budzyński, M.; Wąsik-Szczepanek, E.; Zmorzyński, S.; Karczmarczyk, A.; Giannopoulos, K. Expression of circulating miRNAs associated with lymphocyte differentiation and activation in CLL—another piece in the puzzle. Ann. Hematol. 2017, 96, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Tan, L.P.; Dijkstra, M.K.; van Lom, K.; Robertus, J.L.; Harms, G.; Blokzijl, T.; Kooistra, K.; van T’veer, M.B.; Rosati, S.; et al. miRNA analysis in B-cell chronic lymphocytic leukaemia: Proliferation centres characterized by low miR-150 and high BIC/miR-155 expression. J. Pathol. 2008, 215, 13–20. [Google Scholar] [CrossRef]
- Vigorito, E.; Perks, K.L.; Abreu-Goodger, C.; Bunting, S.; Xiang, Z.; Kohlhaas, S.; Das, P.P.; Miska, E.A.; Rodriguez, A.; Bradley, A.; et al. microRNA-155 Regulates the Generation of Immunoglobulin Class-Switched Plasma Cells. Immunology 2007, 27, 847–859. [Google Scholar] [CrossRef] [Green Version]
- Cui, B.; Chen, L.; Zhang, S.; Mraz, M.; Fecteau, J.-F.; Yu, J.; Ghia, E.M.; Zhang, L.; Bao, L.; Rassenti, L.Z.; et al. MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood 2014, 124, 546–554. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, R.M.; Rao, D.S.; Chaudhuri, A.A.; Boldin, M.P.; Taganov, K.D.; Nicoll, J.; Paquette, R.L.; Baltimore, D. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J. Exp. Med. 2008, 205, 585–594. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Wang, L.-M.; Shen, J.-J. Overexpression of miR-32 inhibits the proliferation and metastasis of ovarian cancer cells by targeting BTLA. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4671–4678. [Google Scholar] [PubMed]
- Stacchini, A.; Aragno, M.; Vallario, A.; Alfarano, A.; Circosta, P.; Gottardi, D.; Faldella, A.; Rege-Cambrin, G.; Thunberg, U.; Nilsson, K.; et al. MEC1 and MEC2: Two new cell lines derived from B-chronic lymphocytic leukaemia in prolymphocytoid transformation. Leuk Res. 1999, 23, 127–136. [Google Scholar] [CrossRef]
- Mainou-Fowler, T.; Proctor, S.J.; Miller, S.; Dickinson, A.M. Expression and production of interleukin 4 in B-cell chronic lymphocytic leukaemia. Leuk. Lymphoma 2001, 42, 689–698. [Google Scholar] [CrossRef]
- Hurdayal, R.; Ndlovu, H.; Revaz-Breton, M.; Parihar, S.P.; Nono, J.K.; Govender, M.; Brombacher, F. IL-4–producing B cells regulate T helper cell dichotomy in type 1- and type 2-controlled diseases. Proc. Natl. Acad. Sci. USA 2017, 114, E8430–E8439. [Google Scholar] [CrossRef] [Green Version]
- Damle, R.N.; Calissano, C.; Chiorazzi, N. Chronic lymphocytic leukaemia: A disease of activated monoclonal B cells. Best Pract. Res. Clin. Haematol. 2010, 23, 33–45. [Google Scholar] [CrossRef] [Green Version]






| Clinical Parameter | Characteristics Value |
|---|---|
| Total number of Patients (n) | 20 |
| Gender (Female/Male) | 6/14 |
| Age | 72.8 ± 9.46 |
| Rai Stage | |
| 0 | 9 |
| 1 | 6 |
| 2 | 2 |
| 3 | 1 |
| 4 | 2 |
| WBC count (1 × 109/L) | 62.4 ± 50.9 |
| Lymphocyte count (1 × 109/L) | 55.2 ± 48.7 |
| Hb level (g/dL) | 13.0 ± 1.5 |
| Platelet count (1 × 109/L) | 185.7 ± 73.8 |
| LDH (U/L) | 168.5 ± 52.5 |
| β2-microglobulin (mg/L) | 3.8 ± 1.7 |
| CD19+ Cells | Frequency of Positive Cells | |||
|---|---|---|---|---|
| CLL Patients | Healthy Controls | |||
| BTLA+IL-4+ | BTLA-IL-4+ | BTLA+IL-4+ | BTLA-IL-4+ | |
| mean | 56.2 | 47.2 | 48.4 | 52.3 |
| median | 54.5 | 41.5 | 56.4 | 51.2 |
| SD | 22.5 | 23.6 | 17.9 | 13.9 |
| range | 19.0–91.0 | 16.0–97.0 | 11.3–72.2 | 33.3–73.0 |
| statistics | BTLA+IL-4+ vs. BTLA-IL-4+ p = NS | BTLA+IL-4+ vs. BTLA-IL-4+ p = NS | ||
| BTLA+IL-4+ CLL vs. HD, p = NS | BTLA-IL-4+ CLL vs. HD, p = NS | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabon, L.; Andrzejczak, A.; Ciszak, L.; Tomkiewicz, A.; Szteblich, A.; Bojarska-Junak, A.; Roliński, J.; Wołowiec, D.; Wróbel, T.; Kosmaczewska, A. BTLA Expression in CLL: Epigenetic Regulation and Impact on CLL B Cell Proliferation and Ability to IL-4 Production. Cells 2021, 10, 3009. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10113009
Karabon L, Andrzejczak A, Ciszak L, Tomkiewicz A, Szteblich A, Bojarska-Junak A, Roliński J, Wołowiec D, Wróbel T, Kosmaczewska A. BTLA Expression in CLL: Epigenetic Regulation and Impact on CLL B Cell Proliferation and Ability to IL-4 Production. Cells. 2021; 10(11):3009. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10113009
Chicago/Turabian StyleKarabon, Lidia, Anna Andrzejczak, Lidia Ciszak, Anna Tomkiewicz, Aleksandra Szteblich, Agnieszka Bojarska-Junak, Jacek Roliński, Dariusz Wołowiec, Tomasz Wróbel, and Agata Kosmaczewska. 2021. "BTLA Expression in CLL: Epigenetic Regulation and Impact on CLL B Cell Proliferation and Ability to IL-4 Production" Cells 10, no. 11: 3009. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10113009