Annexin A1 Mimetic Peptide and Piperlongumine: Anti-Inflammatory Profiles in Endotoxin-Induced Uveitis
Abstract
:1. Introduction
2. Materials and Methods
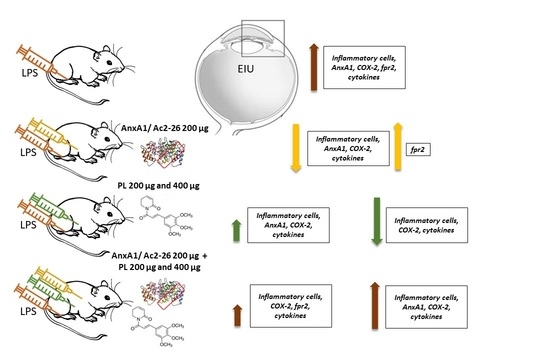
2.1. Experimental Model of Uveitis and Treatment Protocols
2.2. Histopathological and Quantitative Analyses
2.3. Immunohistochemical and Densitometric Studies
2.4. Inflammatory Mediator Levels
2.5. Statistical Analyses
3. Results
3.1. Singly Administered, the Treatments Inhibited the Influx of Leukocytes, Indicating Protective Effects of PL, Especially at 400 μg Dosage, and Confirming the Anti-Inflammatory Action of Ac2-26, but These Effects Were Lost with Coadministration
3.2. Ac2-26 and PL Singly Administered Reduced the Release of Proinflammatory Mediators in EIU, but These Effects Were Abrogated with Coadministration, Especially Ac2-26 + PL 400 μg
3.3. COX-2 Expression Is Not Inhibited after Treatments with PL 200 μg and Ac2-26 + PL 400 μg
3.4. Endogenous AnxA1 Increased during Inflammation in Ocular Tissues, but Ac2-26 Administered Singly or in Combination with PL at Lower Dosage Reduced AnxA1 Immunoreactivity
3.5. Expression of the fpr2 Receptor Is Modulated by Treatment with AnxA1 Peptide but Not with PL Singly Administered or Ac2-26 in Combination with PL 400 µg
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majumder, P.D.; Ghosh, A.; Biswas, J. Infectious uveitis: An enigma. Middle East Afr. J. Ophthalmol. 2017, 24, 2–10. [Google Scholar] [PubMed]
- Mohlin, C.; Sandholm, K.; Ekdahl, K.N.; Nilsson, B. The link between morphology and complement in ocular disease. Mol. Immunol. 2017, 89, 84–99. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Sahawneh, H.F.; Ma, L.; Kubaisi, B.; Schmidt, A.; Foster, C.S. A review and update on orphan drugs for the treatment of noninfectious uveitis. Clin. Ophthalmol. 2017, 11, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, G.F.; Carr, J.M.; Smith, J.R. Emerging infectious uveitis: Chikungunya, dengue, Zika and Ebola: A review. Clin. Exp. Ophthalmol. 2019, 47, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, M.; Desbois, C.; Domont, F.; Maalouf, G.; Touhami, S.; Cacoub, P.; Bodaghi, B.; Saadoun, D. Biotherapies in Uveitis. J. Clin. Med. 2020, 9, 3599. [Google Scholar] [CrossRef]
- Barry, R.J.; Nguyen, Q.D.; Lee, R.W.; Murray, P.I.; Denniston, A.K. Pharmacotherapy for uveitis: Current management and emerging therapy. Clin. Ophthalmol. 2014, 8, 1891–1911. [Google Scholar]
- Chen, S.C.; Sheu, S.J. Recent advances in managing and understanding uveitis. F1000Res 2017, 6, 280. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, A.; Katelaris, C. The Use of Biologic Agents in the Management of Uveitis. Intern. Med. J. 2019, 49, 1352–1363. [Google Scholar] [CrossRef]
- Hassan, M.; Nguyen, N.V.; Halim, M.S.; Afridi, R.; Sadiq, M.A.; Karkhur, S.; Vigil, E.; Karabekirogullari, S.; Nguyen, Q.D.; Do, D.V.; et al. Effect of vitreomacular adhesion on the treatment outcomes in the STOP-Uveitis clinical trial for non-infectious uveitis. J. Ophthalmic Inflamm. Infect. 2019, 9, 12. [Google Scholar] [CrossRef]
- Pleyer, U.; Neri, P.; Deuter, C. New pharmacotherapy options for noninfectious posterior uveitis. Int. Ophthalmol. 2021, 41, 2265–2281. [Google Scholar] [CrossRef]
- Touhami, S.; Diwo, E.; Sève, P.; Trad, S.; Bielefeld, P.; Sène, D.; Abad, S.; Brézin, A.; Quartier, P.; Paut, I.K.; et al. Expert opinion on the use of biological therapy in non-infectious uveitis. Expert Opin. Biol. Ther. 2019, 19, 477–490. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Comstock, T.L.; Cavet, M.E. Impact of the Topical Ophthalmic Corticosteroid Loteprednol Etabonate on Intraocular Pressure. Adv. Ther. 2016, 33, 532–552. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Zhou, D.; Zhang, S.; He, Y.; Lin, Z.; Huang, C.; Li, J. Amelioration of experimental autoimmune uveitis by leflunomide in Lewis rats. PLoS ONE 2013, 8, e62071. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, Z.A.; Asanoma, M.; Iwata, A.; Ishifune, C.; Maekawa, Y.; Shimada, M.; Yasutomo, K. Abrogation of Rbpj Attenuates Experimental Autoimmune Uveoretinitis by Inhibiting IL-22-Producing CD4(+) T Cells. PLoS ONE 2014, 9, e89266. [Google Scholar] [CrossRef]
- Harthan, J.S.; Opitz, D.L.; Fromstein, S.R.; Morettin, C.E. Diagnosis and treatment of anterior uveitis: Optometric management. Clin. Optom. 2016, 8, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, P.S.; Girol, A.P.; Oliani, S.M. Mast cells modulate the inflammatory process in endotoxin-induced uveitis. Mol. Vis. 2011, 17, 1310–1319. [Google Scholar]
- Girol, A.P.; Mimura, K.K.O.; Drewes, C.C.; Bolonheis, S.M.; Solito, E.; Farsky, S.H.P.; Gil, C.D.; Oliani, S.M. Anti-inflammatory mechanisms of the annexin A1 protein and its mimetic peptide Ac2-26 in models of ocular inflammation in vivo and in vitro. J. Immunol. 2013, 190, 5689–5701. [Google Scholar] [CrossRef]
- Yazid, S.; Gardner, P.J.; Carvalho, L.; Chu, C.J.; Flower, R.J.; Solito, E.; Lee, R.W.J.; Ali, R.R.; Dick, A.D. Annexin-A1 restricts Th17 cells and attenuates the severity of autoimmune disease. J. Autoimmun. 2015, 58, 1–11. [Google Scholar] [CrossRef]
- Cardin, L.T.; Sonehara, N.M.; Mimura, K.K.O.; Dos Santos, A.R.D.; da Silva Junior, W.A.; Sobral, L.M.; Leopoldino, A.M.; Cunha, B.R.; Tajara, E.H.; Oliani, S.M.; et al. ANXA1Ac2-26 peptide, a possible therapeutic approach in inflammatory ocular diseases. Gene 2017, 614, 26–36. [Google Scholar] [CrossRef]
- Gardner, P.J.; Yazid, S.; Ribeiro, J.; Ali, R.R.; Dick, A.D. Augmenting Endogenous Levels of Retinal Annexin A1 Suppresses Uveitis in Mice. Transl. Vis. Sci. Technol. 2017, 6, 10. [Google Scholar] [CrossRef]
- Flower, R.; Blackwell, G. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature 1979, 278, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Flower, R.; Gaddum, E. Lipocortin and the mechanism of action of the glucocorticoids. Br. J. Pharmacol. 1988, 94, 987–1015. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.H.; Solito, E. Annexin A1: Uncovering the Many Talents of an Old Protein. Int. J. Mol. Sci. 2018, 19, 1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, G.; Zhou, H.; Zhang, Q.; Jin, Y.; Fu, C. Advancements of Annexin A1 in inflammation and tumorigenesis. Onco Targets Ther. 2019, 12, 3245–3254. [Google Scholar] [CrossRef] [Green Version]
- Lizarbe, M.A.; Barrasa, J.I.; Olmo, N.; Gavilanes, F.; Turnay, J. Annexin-phospholipid interactions. Functional implications. Int. J. Mol. Sci. 2013, 14, 2652–2683. [Google Scholar] [CrossRef] [Green Version]
- Gobbetti, T.; Cooray, S.N. Annexin A1 and resolution of inflammation: Tissue repairing properties and signalling signature. Biol. Chem. 2016, 397, 981–993. [Google Scholar] [CrossRef]
- Grewal, T.; Wason, S.J.; Enrich, C.; Rentero, C. Annexins-insights from knockout mice. Biol. Chem. 2016, 397, 1031–1053. [Google Scholar] [CrossRef]
- Solito, E.; Christian, H.C.; Festa, M.; Mulla, A.; Tierney, T.; Flower, R.J.; Buckingham, J.C. Post-translational modification plays an essential role in the translocation of annexin A1 from the cytoplasm to the cell surface. FASEB J. 2006, 20, 1498–1500. [Google Scholar] [CrossRef]
- Leoni, G.; Nusrat, A. Annexin A1: Shifting the balance towards resolution and repair. Biol. Chem. 2016, 397, 971–979. [Google Scholar] [CrossRef]
- Mimura, K.K.; Tedesco, R.C.; Calabrese, K.S.; Gil, C.D.; Oliani, S.M. The involvement of anti-inflammatory protein, annexin A1, in ocular toxoplasmosis. Mol. Vis. 2012, 18, 1583–1593. [Google Scholar]
- Zanon, C.F.; Sonehara, N.M.; Girol, A.P.; Gil, C.D.; Oliani, S.M. Protective effects of the galectin-1 protein on in vivo and in vitro models of ocular inflammation. Mol. Vis. 2015, 21, 1036–1050. [Google Scholar]
- Gimenes, A.D.; Andrade, T.R.M.; Mello, C.B.; Ramos, L.; Gil, C.D.; Oliani, S.M. Beneficial effect of annexin A1 in a model of experimental allergic conjunctivitis. Exp. Eye Res. 2015, 134, 24–32. [Google Scholar] [CrossRef]
- Jorge, Y.C.; Mataruco, M.M.; Araújo, L.P.; Rossi, A.F.T.; Oliveira, J.G.; Valsechi, M.C.; Caetano, A.; Miyazaki, K.; Fazzio, C.S.J.; Thomé, J.A.; et al. Expression of annexin-A1 and galectin-1 anti-inflammatory proteins and mRNA in chronic gastritis and gastric cancer. Mediat. Inflamm. 2013, 2013, 152860. [Google Scholar] [CrossRef]
- Da Silva, R.A.; Hamade, A.M.A.; Silva, G.A.S.; Pereira, G.H.; De Oliveira, F.F.J.; Costa, S.S.; Iyomasa-Pilon, M.M.; Souza, H.R.; Possebon, L.; Girol, A.P. Evaluation of Annexin A1 Protein in an Infectious Keratitis Model: Therapeutic Perspectives. Curr. Trends Ophthal. 2019, 2, 104–112. [Google Scholar] [CrossRef]
- Marmorato, M.P.; Gimenes, A.D.; Andrade, F.E.C.; Oliani, S.M.; Gil, C.D. Involvement of the annexin A1-Fpr anti-inflammatory system in the ocular allergy. Eur. J. Pharmacol. 2019, 842, 298–305. [Google Scholar] [CrossRef]
- Henrique, T.; Zanon, C.F.; Girol, A.P.; Stefanini, A.C.B.; Contessoto, N.S.A.; Silveira, N.J.F.; Bezerra, D.P.; Silveira, E.R.; Barbosa-Filho, J.M.; Cornélio, M.L.; et al. Biological and physical approaches on the role of piplartine (piperlongumine) in cancer. Sci. Rep. 2020, 10, 22283. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Pessoa, C.; Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef]
- Sun, L.D.; Wang, F.; Dai, F.; Wang, Y.H.; Lin, D.; Zhou, B. Development and mechanism investigation of a new piperlongumine derivative as a potent anti-inflammatory agent. Biochem. Pharmacol. 2015, 95, 156–169. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Historical Spice as a Future Drug: Therapeutic Potential of Piperlongumine. Curr. Pharm. Des. 2016, 22, 4151–4159. [Google Scholar] [CrossRef]
- Meegan, M.J.; Nathwani, S.; Twamley, B.; Zisterer, D.M.; O’Boyle, N.M. Piperlongumine (piplartine) and analogues: Antiproliferative microtubule-destabilising agents. Eur. J. Med. Chem. 2017, 125, 453–463. [Google Scholar] [CrossRef]
- Srivastava, A.; Karthick, T.; Joshi, B.D.; Mishra, R.; Tandon, P.; Ayala, A.P.; Ellena, J. Spectroscopic (far or terahertz, mid-infrared and Raman) investigation, thermal analysis and biological activity of piplartine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 184, 368–381. [Google Scholar] [CrossRef]
- Sant’Ana, M.; Souza, H.R.; Possebon, L.; Cornélio, M.L.; Riffo-Vasquez, Y.; Girol, A.P.; Oliani, S.M. Effect of piperlongumine during exposure to cigarette smoke reduces inflammation and lung injury. Pulm. Pharmacol. Ther. 2020, 61, 10–16. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Castro, F.O.; Alves, A.P.N.N.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Lima, M.A.S.; Elmiro, F.J.M.; Alencar, N.M.N.; Mesquita, R.O.; et al. In vitro and in vivo antitumor effect of 5-FU combined with piplartine and piperine. J. Appl. Toxicol. 2008, 28, 156–163. [Google Scholar] [CrossRef]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F.; et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef]
- Zheng, J.; Son, D.J.; Gu, S.M.; Woo, J.R.; Ham, Y.W.; Lee, H.P.; Kim, W.J.; Jung, J.K.; Hong, J.T. Piperlongumine inhibits lung tumor growth via inhibition of nuclear factor kappa B signaling pathway. Sci. Rep. 2016, 6, 26357. [Google Scholar] [CrossRef]
- Lee, W.; Yoo, H.; Kim, J.A.; Lee, S.; Jee, J.G.; Lee, M.Y.; Lee, Y.M.; Bae, J.S. Barrier protective effects of piperlonguminine in LPS-induced inflammation in vitro and in vivo. Food Chem. Toxicol. 2013, 58, 149–157. [Google Scholar] [CrossRef]
- Seo, Y.H.; Kim, J.K.; Jun, J.G. Synthesis and biological evaluation of piperlongumine derivatives as potent anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2014, 24, 5727–5730. [Google Scholar] [CrossRef]
- He, H.; Guo, W.W.; Xu, R.R.; Chen, X.Q.; Zhang, N.; Wu, X.; Wang, X.M. Alkaloids from piper longum protect dopaminergic neurons against inflammation-mediated damage induced by intranigral injection of lipopolysaccharide. BMC Complement. Altern. Med. 2016, 16, 412. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.M.; Lee, H.P.; Ham, Y.W.; Son, D.J.; Kim, H.Y.; Oh, K.W.; Han, S.; Yun, J.; Hong, J.T. Piperlongumine Improves Lipopolysaccharide-Induced Amyloidogenesis by Suppressing NF-KappaB Pathway. Neuromol. Med. 2018, 20, 312–327. [Google Scholar] [CrossRef] [Green Version]
- Brito, B.; Zamora, D.O.; Bonnah, R.A.; Pan, Y.; Planck, S.R.; Rosenbaum, J.T. Toll-like receptor 4 and CD14 expression in human ciliary body and TLR-4 in human iris endothelial cells. Exp. Eye Res. 2004, 79, 203–208. [Google Scholar] [CrossRef]
- Chang, J.; McCluskey, P.; Wakefield, D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br. J. Ophthalmol. 2006, 90, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Lu, H.; Hu, X.; Chen, W.; Xu, Y.; Wang, J. Expression of TLR4-MyD88 and NF-κB in the iris during endotoxin-induced uveitis. Mediat. Inflamm. 2010, 2010, 748218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Lu, H.; Wang, J.; Qi, X.; Liu, X.; Zhang, X. The effect of toll-like receptor 4 on macrophage cytokines during endotoxin induced uveitis. Int. J. Mol. Sci. 2012, 13, 7508–7520. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Silva, D.; Baroni, S.; Svidzinski, A.E.; Bersani-Amado, C.A.; Cortez, D.A.G. Anti-inflammatory activity of the extract, fractions and amides from the leaves of Piper ovatum Vahl (Piperaceae). J. Ethnopharmacol. 2008, 116, 569–573. [Google Scholar] [CrossRef]
- Yadav, V.; Chatterjee, S.S.; Majeed, M.; Kumar, V. Preventive potentials of piperlongumine and a Piper longum extract against stress responses and pain. J. Tradit. Complement. Med. 2015, 6, 413–423. [Google Scholar]
- Sun, J.; Xu, P.; Du, X.; Zhang, Q.; Zhu, Y. Piperlongumine attenuates collagen-induced arthritis via expansion of myeloid-derived suppressor cells and inhibition of the activation of fibroblast-like synoviocytes. Mol. Med. Rep. 2015, 11, 2689–2694. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, J.E.; Pepple, K.L. Cytokines in uveitis. Curr. Opin. Ophthalmol. 2018, 29, 267–274. [Google Scholar] [CrossRef]
- Balamurugan, S.; Das, D.; Hasanreisoglu, M.; Toy, B.C.; Akhter, M.; Anuradha, V.K.; Anthony, E.; Gurnani, B.; Kaur, K. Interleukins and cytokine biomarkers in uveitis. Indian J. Ophthalmol. 2020, 68, 1750–1763. [Google Scholar] [CrossRef]
- Chen, W.; Hu, W.F.; Zhao, L.; Li, S.; Lu, H. Toll-like receptor 4 expression in macrophages in endotoxin-induced uveitis in Wistar rats. Chin. J. Ophthalmol. 2010, 46, 355–361. [Google Scholar]
- de Coupade, C.; Ajuebor, M.N.; Russo-Marie, F.; Perretti, M.; Solito, E. Cytokine modulation of liver annexin 1 expression during experimental endotoxemia. Am. J. Pathol. 2001, 159, 1435–1443. [Google Scholar] [CrossRef] [Green Version]
- Damazo, A.; Flower, R.J.; Solito, E.; Oliani, S.M. Annexin-A1 gene expression during liver development and post-translation modification after experimental endotoxemia. Inflamm. Res. 2008, 57, 97–103. [Google Scholar] [CrossRef]
- Da Cunha, E.E.; OlianI, S.M.; Damazo, A.S. Effect of annexin-A1 peptide treatment during lung inflammation induced by lipopolysaccharide. Pulm. Pharmacol. Ther. 2012, 25, 303–311. [Google Scholar] [CrossRef]
- Vago, J.P.; Tavares, L.P.; Garcia, C.C.; Lima, K.M.; Perucci, L.O.; Vieira, É.L.; Nogueira, C.R.; Soriani, F.M.; Martins, J.O.; Silva, P.M.; et al. The role and effects of glucocorticoid-induced leucine zipper in the context of inflammation resolution. J. Immunol. 2015, 194, 4940–4950. [Google Scholar] [CrossRef] [Green Version]
- He, X.D.; Wang, Y.; Wu, Q.; Wang, H.X.; Chen, Z.D.; Zheng, R.S.; Wang, Z.S.; Wang, J.B.; Yang, Y. Xuebijing Protects Rats from Sepsis Challenged with Acinetobacter baumannii by Promoting Annexin A1 Expression and Inhibiting Proinflammatory Cytokines Secretion. Evid. Based Complement. Altern. Med. 2013, 2013, 804940. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.Y.; Jin, Y.; Mao, J.T.; Zhang, Z.F.; Heber, D.; Dubinett, S.M.; Rao, J. Green tea inhibits cycolooxygenase-2 in non-small cell lung cancer cells through the induction of Annexin-1. Biochem. Biophys. Res. Commun. 2012, 427, 725–730. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girol, A.P.; de Freitas Zanon, C.; Caruso, Í.P.; de Souza Costa, S.; Souza, H.R.; Cornélio, M.L.; Oliani, S.M. Annexin A1 Mimetic Peptide and Piperlongumine: Anti-Inflammatory Profiles in Endotoxin-Induced Uveitis. Cells 2021, 10, 3170. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10113170
Girol AP, de Freitas Zanon C, Caruso ÍP, de Souza Costa S, Souza HR, Cornélio ML, Oliani SM. Annexin A1 Mimetic Peptide and Piperlongumine: Anti-Inflammatory Profiles in Endotoxin-Induced Uveitis. Cells. 2021; 10(11):3170. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10113170
Chicago/Turabian StyleGirol, Ana Paula, Caroline de Freitas Zanon, Ícaro Putinhon Caruso, Sara de Souza Costa, Helena Ribeiro Souza, Marinônio Lopes Cornélio, and Sonia Maria Oliani. 2021. "Annexin A1 Mimetic Peptide and Piperlongumine: Anti-Inflammatory Profiles in Endotoxin-Induced Uveitis" Cells 10, no. 11: 3170. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10113170