Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction
Abstract
:1. Introduction
2. G Protein-Coupled Receptors (GPCRs)
2.1. Structure
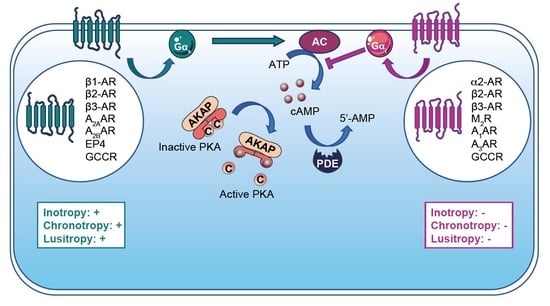
2.2. Adrenoreceptors
2.2.1. α-Adrenergic Receptors (α-AR)
2.2.2. β-Adrenergic Receptors (β-AR)
2.3. Miscellaneous Cardiac GPCRs
2.3.1. Muscarinic Receptors Type 2
2.3.2. Adenosine Receptors (or P1 Receptors)
2.3.3. Prostaglandin Receptors
2.3.4. Glucagon and Glucagon Like Petide-1 Receptors (GCCR and GLP1R)
3. Adenylyl Cyclases
3.1. Structure and Function
3.2. Role of Major ACs in Cardiac Physiology and During MI
3.3. Miscellaneous Cardiac ACs
4. Cyclic-AMP Downstream Effectors
4.1. Protein Kinase-A or cAMP-Dependent Protein Kinase (PKA)
4.1.1. Structure
4.1.2. PKA Modulates Cardiac Function
4.1.3. PKA in Myocardial Infarction
4.2. Epac (Exchange Protein Activated by cAMP)
4.2.1. Epac Structure
4.2.2. Epac Cardiac Function
4.2.3. Epac in Myocardial Infarction
4.3. Cyclic Nucleotide-Regulated Cations Channels (CNCC)
4.4. Popeye Domain-Containing Protein
5. A-Kinase Anchoring Proteins (AKAP)
5.1. Structure and Function
5.2. Cardiac AKAPs with Patophysiological Function in MI
5.3. Miscellaneous Cardiac AKAPs
6. Phosphodiesterases (PDEs)
6.1. Structure and Function
6.2. Cardiac PDEs with Physiological Function in MI
6.3. Miscellaneous Cardiac PDEs
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-AR | Alpha adrenergic receptor |
| α1-AR | Alpha 1 adrenergic receptor |
| α2-AR | Alpha 2 adrenergic receptor |
| α-SMA | Alpha smooth muscle actinin |
| A2AR | Adenosine receptor A2 |
| A2BAR | Adenosine receptor A2B |
| A3AR | Adenosine receptor A3A |
| AC | Adenylyl cyclase |
| ACE | Angiotensin converting enzyme |
| ACS | Acute coronary syndrome |
| AKAP | A-kinase anchoring protein |
| AMI | Acute myocardial infarction |
| ANG II | Angiotensin II |
| AV block | Atrio ventricular block |
| BAD | Bcl2 associated death promoter |
| β-AR | Beta adrenergic receptor |
| β1-AR | Beta 1 adrenergic receptor |
| β2-AR | Beta 2 adrenergic receptor |
| β3-AR | Beta 3 adrenergic receptor |
| βARK1 | Beta adrenergic receptor kinase 1 |
| β-Ars | Beta arrestins |
| CAMKII | Ca2+/Calmodulin-dependent protein kinase II |
| cAMP | Cyclic adenosine 3′-5′-monophosphate |
| CaN | Calcineurin |
| CEC | Cardiac excitation–contraction coupling |
| CF | Cardiac fibroblasts |
| cGMP | Cyclic guanosine-3′,5′-monophosphate |
| CHD | Coronary heart diseases |
| CHID | Chronic ischemic heart disease |
| CICR | Calcium-induced calcium release |
| CNCC | Cyclic nucleotide regulates cations channel |
| CNGC | Cyclic nucleotide-gated channels |
| CNG | Cyclic nucleotide-gated ion channels |
| cNMP | Cyclic nucleotide-3′,5′-monophosphate |
| CTGF | Connective tissue growth factor |
| cTnT | Cardiac Troponin T |
| Drp1 | GTPase dynamin related protein 1 |
| eNOS | Endothelial NO synthase |
| EP3 | Prostaglandin EP3 receptor |
| EP4 | Prostaglandin EP4 receptor |
| Epac | Exchange protein activated by cAMP |
| ERK5 | Extracellular signal-regulated kinase 5 |
| FIS1 | Mitochondrial fission 1 protein |
| GCCR | Glucagon receptor |
| GEF | Guanosine exchange factor |
| GLP1 | Glucagon-like peptide 1 |
| GLP1R | Glucagon-like peptide 1 receptor |
| GPCR | G protein-coupled receptor |
| GRK2 | G protein-coupled receptor kinase 2 |
| GSK3β | Glycogen synthase kinase 3 beta |
| HCN | Hyperpolarization-activated cyclic nucleotide–gated channels |
| HF | Heart failure |
| HIF1α | Hypoxia inducible factor 1alpha |
| HR | Heart rate |
| I/R | Ischemia/reperfusion |
| ICEF | Ischemia-induced caveolin 3 enriched fractions |
| ICER | Inducible cAMP early repressor |
| KCNQ1 | Potassium voltage-gated channel subfamily-Q member 1 |
| LAD | Left anterior descending artery ligation |
| LTCC | L-type calcium channel |
| M2R | Muscarinic receptor type 2 |
| MEF2 | Myocyte enhancer factor 2 |
| MEK5 | Mitogen-activated protein kinase kinase 5 |
| MI | Myocardial infarction |
| miR-21 | miRNA-21 |
| MKK3 | Mitogen-activated protein kinase 3 |
| MLTK | MLK-like mitogen-activated protein triple kinase |
| mPTP | Mitochondrial permeability transition pore |
| MSCs | Mesenchymal stem cells |
| MyBPC | Myosin binding protein C |
| NAC | N acetyl cysteine |
| NCX | Na/Ca exchanger |
| NFATc3 | Nuclear factor of activated T-cells, cytoplasmic 3 |
| NO | Nitric oxide |
| PDE | Phosphodiesterase |
| PDK1 | Pyruvate dehydrogenase kinase 1 |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphatidyl inositol-3 kinase |
| PKA | Protein kinase-A |
| PKC | Protein kinase-C |
| PKD | Protein kinase-D |
| PKD1 | Polycystic kidney disease 1 |
| PKG | Protein kinase G |
| PKI | Protein kinase-A inhibitor |
| PLB | Phospholamban |
| PLC | Phospholipase C |
| PLK1 | Polo-like kinase1 |
| POPDC | Popeye domain containing protein |
| PP1 | Protein phosphatase 1 |
| PP2B | Protein phosphatase 2B |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| PPI disruptors | Protein–protein interaction disruptors |
| PPs | Protein phosphatases |
| ROS | Reactive oxygen species |
| RSK3 | Ribosomal S6 kinase |
| RyR | Ryanodin receptor |
| sAC | Soluble adenylyl cyclase |
| SAN | Sino atrial node |
| SCD | Sudden cardiac death |
| SERCA2 | Sarco/endoplasmic reticulum Ca2+-ATPase |
| Siah2 | Ubiquitin protein ligase seven in absentia homolog 2 |
| SKIP | Sphingosine kinase type 1-interacting protein |
| smAKAP | Small membrane A-kinase anchoring protein |
| SNP | Single-nucleotide polymorphism |
| SR | Sarcoplasmic reticulum |
| STEMI | ST elevation myocardial infarction |
| TAC | Transverse aortic constriction |
| TnI | Troponin I |
References
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and Endocrine Control of Cardiovascular Function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Stress-Activated Cytokines and The Heart: From Adaptation to Maladaptation. Annu. Rev. Physiol. 2003, 65, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Boularan, C.; Gales, C. Cardiac CAMP: Production, Hydrolysis, Modulation and Detection. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pidoux, G.; Taskén, K. Specificity and Spatial Dynamics of Protein Kinase A Signaling Organized by A-Kinase-Anchoring Proteins. J. Mol. Endocrinol. 2010, 44, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Chao, Y.-C.; Surdo, N.C.; Pantano, S.; Zaccolo, M. Imaging CAMP Nanodomains in the Heart. Biochem. Soc. Trans. 2019, 47, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Corbin, J.D.; Sugden, P.H.; Lincoln, T.M.; Keely, S.L. Compartmentalization of Adenosine 3’:5’-Monophosphate and Adenosine 3’:5’-Monophosphate-Dependent Protein Kinase in Heart Tissue. J. Biol. Chem. 1977, 252, 3854–3861. [Google Scholar] [CrossRef]
- Brunton, L.L.; Hayes, J.S.; Mayer, S.E. Hormonally Specific Phosphorylation of Cardiac Troponin I and Activation of Glycogen Phosphorylase. Nature 1979, 280, 78–80. [Google Scholar] [CrossRef]
- Hayes, J.S.; Brunton, L.L.; Brown, J.H.; Reese, J.B.; Mayer, S.E. Hormonally Specific Expression of Cardiac Protein Kinase Activity. Proc. Natl. Acad. Sci. USA 1979, 76, 1570–1574. [Google Scholar] [CrossRef] [Green Version]
- Buxton, I.L.; Brunton, L.L. Compartments of Cyclic AMP and Protein Kinase in Mammalian Cardiomyocytes. J. Biol. Chem. 1983, 258, 10233–10239. [Google Scholar] [CrossRef]
- Xiao, R.P.; Lakatta, E.G. Beta 1-Adrenoceptor Stimulation and Beta 2-Adrenoceptor Stimulation Differ in Their Effects on Contraction, Cytosolic Ca2+, and Ca2+ Current in Single Rat Ventricular Cells. Circ. Res. 1993, 73, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, V.; Pak, E.; Robinson, R.B.; Steinberg, S.F. Beta 2-Adrenergic Receptor Actions in Neonatal and Adult Rat Ventricular Myocytes. Circ. Res. 1995, 76, 40–52. [Google Scholar] [CrossRef]
- Farah, A.E. Glucagon and the Circulation. Pharmacol. Rev. 1983, 35, 181–217. [Google Scholar]
- Vila Petroff, M.G.; Egan, J.M.; Wang, X.; Sollott, S.J. Glucagon-like Peptide-1 Increases CAMP but Fails to Augment Contraction in Adult Rat Cardiac Myocytes. Circ. Res. 2001, 89, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Fischmeister, R.; Castro, L.R.V.; Abi-Gerges, A.; Rochais, F.; Jurevicius, J.; Leroy, J.; Vandecasteele, G. Compartmentation of Cyclic Nucleotide Signaling in the Heart: The Role of Cyclic Nucleotide Phosphodiesterases. Circ. Res. 2006, 99, 816–828. [Google Scholar] [CrossRef] [Green Version]
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–579. [Google Scholar] [CrossRef] [Green Version]
- Boudonas, G.E. β-Blockers in Coronary Artery Disease Management. Hippokratia 2010, 14, 231–235. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task. Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Gabriel-Costa, D. The Pathophysiology of Myocardial Infarction-Induced Heart Failure. Pathophysiology 2018, 25, 277–284. [Google Scholar] [CrossRef]
- Francis, G.S. Pathophysiology of Chronic Heart Failure. Am. J. Med. 2001, 110 (Suppl 7A), 37S–46S. [Google Scholar] [CrossRef]
- Effect of Carvedilol on Mortality and Morbidity in Patients with Chronic Heart Failure. Circulation 1996, 94, 592. [CrossRef]
- López-Sendón, J.; Swedberg, K.; McMurray, J.; Tamargo, J.; Maggioni, A.P.; Dargie, H.; Tendera, M.; Waagstein, F.; Kjekshus, J.; Lechat, P.; et al. Expert Consensus Document on Beta-Adrenergic Receptor Blockers. Eur. Heart J. 2004, 25, 1341–1362. [Google Scholar] [CrossRef]
- Kezerashvili, A.; Marzo, K.; De Leon, J. Beta Blocker Use after Acute Myocardial Infarction in the Patient with Normal Systolic Function: When Is It “Ok” to Discontinue? Curr. Cardiol. Rev. 2012, 8, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Stewart, S.; Jenkins, A.; Buchan, S.; McGuire, A.; Capewell, S.; McMurray, J.J.J.V. The Current Cost of Heart Failure to the National Health Service in the UK. Eur. J. Heart Fail. 2002, 4, 361–371. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the Impact of Heart Failure in the United States: A Policy Statement from the American Heart Association. Circ. Heart. Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [Green Version]
- Hampton, J.R. Choosing the Right β-Blocker. Drugs 1994, 48, 549–568. [Google Scholar] [CrossRef]
- Ko, D.T.; Hebert, P.R.; Coffey, C.S.; Curtis, J.P.; Foody, J.M.; Sedrakyan, A.; Krumholz, H.M. Adverse Effects of Beta-Blocker Therapy for Patients with Heart Failure: A Quantitative Overview of Randomized Trials. Arch. Intern. Med. 2004, 164, 1389–1394. [Google Scholar] [CrossRef] [Green Version]
- Arnold, S.V.; Spertus, J.A.; Lipska, K.J.; Lanfear, D.E.; Tang, F.; Grodzinsky, A.; McGuire, D.K.; Gore, M.O.; Goyal, A.; Maddox, T.M.; et al. Type of Beta-Blocker Use Among Patients with Versus without Diabetes after Myocardial Infarction. Am. Heart J. 2014, 168, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Koren, G.; Norton, G.; Radinsky, K.; Shalev, V. Chronic Use of β-Blockers and the Risk of Parkinson’s Disease. Clin. Drug Investig. 2019, 39, 463–468. [Google Scholar] [CrossRef]
- Zhang, X.; Szeto, C.; Gao, E.; Tang, M.; Jin, J.; Fu, Q.; Makarewich, C.; Ai, X.; Li, Y.; Tang, A.; et al. Cardiotoxic and Cardioprotective Features of Chronic β-Adrenergic Signaling. Circ. Res. 2013, 112, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Whelan, R.S.; Konstantinidis, K.; Xiao, R.-P.; Kitsis, R.N. Cardiomyocyte Life-Death Decisions in Response to Chronic β-Adrenergic Signaling. Circ. Res. 2013, 112, 408–410. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.E.; Kass, D.A. Cardiac Phosphodiesterases and Their Modulation for Treating Heart Disease. Handb. Exp. Pharmacol. 2017, 243, 249–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dema, A.; Perets, E.; Schulz, M.S.; Deák, V.A.; Klussmann, E. Pharmacological Targeting of AKAP-Directed Compartmentalized CAMP Signalling. Cell Signal. 2015, 27, 2474–2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziyatdinova, N.I.; Kuptsova, A.M.; Faskhutdinov, L.I.; Zefirov, A.L.; Zefirov, T.L. Effect of A2-Adrenoceptor Stimulation on Functional Parameters of Langendorff-Isolated Rat Heart. Bull. Exp. Biol. Med. 2018, 165, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Arnar, D.O.; Xing, D.; Martins, J.B. Alpha-2 Adrenergic Antagonism Enhances Risk of Ventricular Tachycardia during Acute Ischemia. Scand. Cardiovasc. J. 2007, 41, 378–385. [Google Scholar] [CrossRef]
- Agarwal, S.R.; Ostrom, R.S.; Harvey, R.D. Membrane Microdomains and cAMP Compartmentation in Cardiac Myocytes. In Microdomains in the Cardiovascular System; Nikolaev, V., Zaccolo, M., Eds.; Cardiac and Vascular Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 17–35. ISBN 978-3-319-54579-0. [Google Scholar]
- Yoo, B.; Lemaire, A.; Mangmool, S.; Wolf, M.J.; Curcio, A.; Mao, L.; Rockman, H.A. B1-Adrenergic Receptors Stimulate Cardiac Contractility and CaMKII Activation in Vivo and Enhance Cardiac Dysfunction Following Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1377–H1386. [Google Scholar] [CrossRef] [Green Version]
- Kompa, A.R.; Gu, X.; Evans, B.A.; Summers, R.J. Desensitization of Cardiac β -Adrenoceptor Signaling with Heart Failure Produced by Myocardial Infarction in the Rat. Evidence for the Role of Gi but Not Gs or Phosphorylating Proteins. J. Mol. Cell. Cardiol. 1999, 31, 1185–1201. [Google Scholar] [CrossRef]
- Bhushan, S.; Kondo, K.; Predmore, B.L.; Zlatopolsky, M.; King, A.L.; Pearce, C.; Huang, H.; Tao, Y.-X.; Condit, M.E.; Lefer, D.J. Selective Β2-Adrenoreceptor Stimulation Attenuates Myocardial Cell Death and Preserves Cardiac Function after Ischemia-Reperfusion Injury. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1865–1874. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Miao, B.; Charles, E.; Kron, I.L.; French, B.A.; Yang, Z. Stimulation of the Beta2 Adrenergic Receptor at Reperfusion Limits Myocardial Reperfusion Injury via IL-10 Dependent Anti-Inflammatory Pathway in the Spleen. Circ. J. 2018, 82, 2829–2836. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, C.; Tavernier, G.; Charpentier, F.; Langin, D.; Le Marec, H. Functional Beta3-Adrenoceptor in the Human Heart. J. Clin. Investig. 1996, 98, 556–562. [Google Scholar] [CrossRef]
- Niu, X.; Zhao, L.; Li, X.; Xue, Y.; Wang, B.; Lv, Z.; Chen, J.; Sun, D.; Zheng, Q. Β3-Adrenoreceptor Stimulation Protects against Myocardial Infarction Injury via ENOS and NNOS Activation. PLoS ONE 2014, 9, e98713. [Google Scholar] [CrossRef] [Green Version]
- García-Prieto, J.; García-Ruiz, J.M.; Sanz-Rosa, D.; Pun, A.; García-Alvarez, A.; Davidson, S.M.; Fernández-Friera, L.; Nuno-Ayala, M.; Fernández-Jiménez, R.; Bernal, J.A.; et al. B3 Adrenergic Receptor Selective Stimulation during Ischemia/Reperfusion Improves Cardiac Function in Translational Models through Inhibition of MPTP Opening in Cardiomyocytes. Basic Res. Cardiol. 2014, 109, 422. [Google Scholar] [CrossRef]
- Mazzadi, A.N.; Pineau, J.; Costes, N.; Le Bars, D.; Bonnefoi, F.; Croisille, P.; Porcher, R.; Chevalier, P. Muscarinic Receptor Upregulation in Patients With Myocardial Infarction: A New Paradigm. Circ. Cardiovasc. Imaging 2009, 2, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Headrick, J.P.; Lasley, R.D. Adenosine Receptors and Reperfusion Injury of the Heart. In Adenosine Receptors in Health and Disease; Wilson, C.N., Mustafa, S.J., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; Volume 193, pp. 189–214. ISBN 978-3-540-89614-2. [Google Scholar]
- Forman, M.B.; Stone, G.W.; Jackson, E.K. Role of Adenosine as Adjunctive Therapy in Acute Myocardial Infarction. Cardiovasc. Drug Rev. 2006, 24, 116–147. [Google Scholar] [CrossRef]
- Bryson, T.D.; Gu, X.; Khalil, R.M.; Khan, S.; Zhu, L.; Xu, J.; Peterson, E.; Yang, X.-P.; Harding, P. Overexpression of Prostaglandin E2 EP4 Receptor Improves Cardiac Function after Myocardial Infarction. J. Mol. Cell Cardiol. 2018, 118, 1–12. [Google Scholar] [CrossRef]
- Zacharowski, K.; Olbrich, A.; Piper, J.; Hafner, G.; Kondo, K.; Thiemermann, C. Selective Activation of the Prostanoid EP 3 Receptor Reduces Myocardial Infarct Size in Rodents. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2141–2147. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Muñoz, C.; Nieto-Cerón, S.; Cabezas-Herrera, J.; Hernández-Cascales, J. Glucagon Increases Contractility in Ventricle but Not in Atrium of the Rat Heart. Eur. J. Pharmacol. 2008, 587, 243–247. [Google Scholar] [CrossRef]
- Ali, S.; Ussher, J.R.; Baggio, L.L.; Kabir, M.G.; Charron, M.J.; Ilkayeva, O.; Newgard, C.B.; Drucker, D.J. Cardiomyocyte Glucagon Receptor Signaling Modulates Outcomes in Mice with Experimental Myocardial Infarction. Mol. Metab. 2015, 4, 132–143. [Google Scholar] [CrossRef]
- Bose, A.K.; Mocanu, M.M.; Carr, R.D.; Brand, C.L.; Yellon, D.M. Glucagon-like Peptide 1 Can Directly Protect the Heart Against Ischemia/Reperfusion Injury. Diabetes 2005, 54, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Noyan-Ashraf, M.H.; Momen, M.A.; Ban, K.; Sadi, A.-M.; Zhou, Y.-Q.; Riazi, A.M.; Baggio, L.L.; Henkelman, R.M.; Husain, M.; Drucker, D.J. GLP-1R Agonist Liraglutide Activates Cytoprotective Pathways and Improves Outcomes After Experimental Myocardial Infarction in Mice. Diabetes 2009, 58, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and Dynamics of GPCR Signaling Complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12. [Google Scholar] [CrossRef]
- Kaykı-Mutlu, G.; Papazisi, O.; Palmen, M.; Danser, A.H.J.; Michel, M.C.; Arioglu-Inan, E. Cardiac and Vascular A1-Adrenoceptors in Congestive Heart Failure: A Systematic Review. Cells 2020, 9, 2412. [Google Scholar] [CrossRef] [PubMed]
- Giovannitti, J.A.; Thoms, S.M.; Crawford, J.J. Alpha-2 Adrenergic Receptor Agonists: A Review of Current Clinical Applications. Anesth. Prog. 2015, 62, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irannejad, R.; Tomshine, J.C.; Tomshine, J.R.; Chevalier, M.; Mahoney, J.P.; Steyaert, J.; Rasmussen, S.G.F.; Sunahara, R.K.; El-Samad, H.; Huang, B.; et al. Conformational Biosensors Reveal GPCR Signalling from Endosomes. Nature 2013, 495, 534–538. [Google Scholar] [CrossRef] [Green Version]
- Irannejad, R.; Pessino, V.; Mika, D.; Huang, B.; Wedegaertner, P.B.; Conti, M.; von Zastrow, M. Functional Selectivity of GPCR-Directed Drug Action through Location Bias. Nat. Chem. Biol. 2017, 13, 799–806. [Google Scholar] [CrossRef]
- Tsvetanova, N.G.; von Zastrow, M. Spatial Encoding of Cyclic AMP Signaling Specificity by GPCR Endocytosis. Nat. Chem. Biol. 2014, 10, 1061–1065. [Google Scholar] [CrossRef]
- Arnold, A.-S.; Tang, Y.L.; Qian, K.; Shen, L.; Valencia, V.; Phillips, M.I.; Zhang, Y.C. Specific Β1-Adrenergic Receptor Silencing with Small Interfering RNA Lowers High Blood Pressure and Improves Cardiac Function in Myocardial Ischemia. J. Hypertens. 2007, 25, 197–205. [Google Scholar] [CrossRef]
- Nikolaev, V.O.; Moshkov, A.; Lyon, A.R.; Miragoli, M.; Novak, P.; Paur, H.; Lohse, M.J.; Korchev, Y.E.; Harding, S.E.; Gorelik, J. Β2-Adrenergic Receptor Redistribution in Heart Failure Changes CAMP Compartmentation. Science 2010, 327, 1653–1657. [Google Scholar] [CrossRef]
- El-Armouche, A.; Eschenhagen, T. Beta-Adrenergic Stimulation and Myocardial Function in the Failing Heart. Heart Fail. Rev. 2009, 14, 225–241. [Google Scholar] [CrossRef]
- Miao, Y.; Li, M.; Wang, C.; Li, H.; Chen, H. Effect of β-Adrenergic Receptor Kinase Inhibitor on Post-Myocardial Infarction Heart Failure in Rats. Int. J. Clin. Exp. Pathol. 2017, 10, 9858–9865. [Google Scholar]
- Zee, R.Y.L.; Cook, N.R.; Reynolds, R.; Cheng, S.; Ridker, P.M. Haplotype Analysis of the Beta2 Adrenergic Receptor Gene and Risk of Myocardial Infarction in Humans. Genetics 2005, 169, 1583–1587. [Google Scholar] [CrossRef] [Green Version]
- Schürks, M.; Kurth, T.; Ridker, P.M.; Buring, J.E.; Zee, R.Y.L. Association between Polymorphisms in the Β2-Adrenergic Receptor Gene with Myocardial Infarction and Ischaemic Stroke in Women. Thromb Haemost 2009, 101, 351–358. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Heckbert, S.R.; Sotoodehnia, N.; Bis, J.C.; Smith, N.L.; Marciante, K.D.; Hindorff, L.A.; Lange, L.A.; Lumley, T.S.; Rice, K.M.; et al. Β1- and Β2-Adrenergic Receptor Gene Variation, β-Blocker Use and Risk of Myocardial Infarction and Stroke. Am. J. Hypertens 2008, 21, 290–296. [Google Scholar] [CrossRef] [Green Version]
- Hautala, A.J.; Tulppo, M.P.; Kiviniemi, A.M.; Rankinen, T.; Bouchard, C.; Mäkikallio, T.H.; Huikuri, H.V. Acetylcholine Receptor M2 Gene Variants, Heart Rate Recovery, and Risk of Cardiac Death after an Acute Myocardial Infarction. Ann. Med. 2009, 41, 197–207. [Google Scholar] [CrossRef]
- Xiao, C.-Y.; Yuhki, K.; Hara, A.; Fujino, T.; Kuriyama, S.; Yamada, T.; Takayama, K.; Takahata, O.; Karibe, H.; Taniguchi, T.; et al. Prostaglandin E 2 Protects the Heart From Ischemia-Reperfusion Injury via Its Receptor Subtype EP 4. Circulation 2004, 109, 2462–2468. [Google Scholar] [CrossRef] [Green Version]
- Steegborn, C. Structure, Mechanism, and Regulation of Soluble Adenylyl Cyclases—Similarities and Differences to Transmembrane Adenylyl Cyclases. Biochim. Biophys. Acta 2014, 1842, 2535–2547. [Google Scholar] [CrossRef] [Green Version]
- Sadana, R.; Dessauer, C.W. Physiological Roles for G Protein-Regulated Adenylyl Cyclase Isoforms: Insights from Knockout and Overexpression Studies. Neurosignals 2009, 17, 5–22. [Google Scholar] [CrossRef]
- Schirmer, I.; Bualeong, T.; Budde, H.; Cimiotti, D.; Appukuttan, A.; Klein, N.; Steinwascher, P.; Reusch, P.; Mügge, A.; Meyer, R.; et al. Soluble Adenylyl Cyclase: A Novel Player in Cardiac Hypertrophy Induced by Isoprenaline or Pressure Overload. PLoS ONE 2018, 13, e0192322. [Google Scholar] [CrossRef] [Green Version]
- Mougenot, N.; Mika, D.; Czibik, G.; Marcos, E.; Abid, S.; Houssaini, A.; Vallin, B.; Guellich, A.; Mehel, H.; Sawaki, D.; et al. Cardiac Adenylyl Cyclase Overexpression Precipitates and Aggravates Age-Related Myocardial Dysfunction. Cardiovasc. Res. 2019, 115, 1778–1790. [Google Scholar] [CrossRef]
- Tang, T.; Lai, N.C.; Roth, D.M.; Drumm, J.; Guo, T.; Lee, K.-W.; Han, P.-L.; Dalton, N.; Gao, M.H. Adenylyl Cyclase Type V Deletion Increases Basal Left Ventricular Function and Reduces Left Ventricular Contractile Responsiveness to Beta-Adrenergic Stimulation. Basic Res. Cardiol. 2006, 101, 117–126. [Google Scholar] [CrossRef]
- Tang, T.; Gao, M.H.; Lai, N.C.; Firth, A.L.; Takahashi, T.; Guo, T.; Yuan, J.X.-J.; Roth, D.M.; Hammond, H.K. Adenylyl Cyclase Type 6 Deletion Decreases Left Ventricular Function via Impaired Calcium Handling. Circulation 2008, 117, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timofeyev, V.; Myers, R.E.; Kim, H.J.; Woltz, R.L.; Sirish, P.; Heiserman, J.P.; Li, N.; Singapuri, A.; Tang, T.; Yarov-Yarovoy, V.; et al. Adenylyl Cyclase Subtype-Specific Compartmentalization: Differential Regulation of L-Type Ca2+ Current in Ventricular Myocytes. Circ. Res. 2013, 112, 1567–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, S.; Takagi, G.; Kawabe, J.; Yang, G.; Lee, M.-C.; Hong, C.; Liu, J.; Vatner, D.E.; Sadoshima, J.; Vatner, S.F.; et al. Disruption of Type 5 Adenylyl Cyclase Gene Preserves Cardiac Function against Pressure Overload. Proc. Natl. Acad. Sci. USA 2003, 100, 9986–9990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, S.; Kawabe, J.; Yatani, A.; Takagi, G.; Lee, M.-C.; Hong, C.; Liu, J.; Takagi, I.; Sadoshima, J.; Vatner, D.E.; et al. Type 5 Adenylyl Cyclase Disruption Alters Not Only Sympathetic But Also Parasympathetic and Calcium-Mediated Cardiac Regulation. Circ. Res. 2003, 93, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bull Melsom, C.; Cosson, M.-V.; Ørstavik, Ø.; Lai, N.C.; Hammond, H.K.; Osnes, J.-B.; Skomedal, T.; Nikolaev, V.; Levy, F.O.; Krobert, K.A. Constitutive Inhibitory G Protein Activity upon Adenylyl Cyclase-Dependent Cardiac Contractility Is Limited to Adenylyl Cyclase Type 6. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Boucher, M.; Nim, S.; de Montigny, C.; Rousseau, G. Alterations of β-Adrenoceptor Responsiveness in Postischemic Myocardium after 72 h of Reperfusion. Eur. J. Pharmacol. 2004, 495, 185–191. [Google Scholar] [CrossRef]
- Bräunig, J.H.; Albrecht-Küpper, B.; Seifert, R. Adenylyl Cyclase Regulation in Heart Failure Due to Myocardial Infarction in Rats. Naunyn-Schmiedeberg’s Arch. Pharm. 2014, 387, 389–398. [Google Scholar] [CrossRef]
- Ma, Y.; Iyer, R.P.; Jung, M.; Czubryt, M.P.; Lindsey, M.L. Cardiac Fibroblast Activation Post-Myocardial Infarction: Current Knowledge Gaps. Trends Pharmacol. Sci. 2017, 38, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Swaney, J.S.; Patel, H.H.; Yokoyama, U.; Lai, N.C.; Spellman, M.; Insel, P.A.; Roth, D.M. Adenylyl Cyclase Activity and Function Are Decreased in Rat Cardiac Fibroblasts after Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3216–H3220. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, T.A.; Dessauer, C.W. Function of Adenylyl Cyclase in Heart: The AKAP Connection. J. Cardiovasc Dev. Dis. 2018, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Bravo, C.A.; Vatner, D.E.; Pachon, R.; Zhang, J.; Vatner, S.F. A Food and Drug Administration–Approved Antiviral Agent That Inhibits Adenylyl Cyclase Type 5 Protects the Ischemic Heart Even When Administered after Reperfusion. J. Pharm. Exp. 2016, 357, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Levy, D.; Oydanich, M.; Bravo, C.A.; Yoon, S.; Vatner, D.E.; Vatner, S.F. A Novel Adenylyl Cyclase Type 5 Inhibitor That Reduces Myocardial Infarct Size Even When Administered after Coronary Artery Reperfusion. J. Mol. Cell. Cardiol. 2018, 121, 13–15. [Google Scholar] [CrossRef]
- Seifert, R. Vidarabine Is Neither a Potent nor a Selective AC5 Inhibitor. Biochem. Pharm. 2014, 87, 543–546. [Google Scholar] [CrossRef]
- Appukuttan, A.; Kasseckert, S.A.; Micoogullari, M.; Flacke, J.-P.; Kumar, S.; Woste, A.; Abdallah, Y.; Pott, L.; Reusch, H.P.; Ladilov, Y. Type 10 Adenylyl Cyclase Mediates Mitochondrial Bax Translocation and Apoptosis of Adult Rat Cardiomyocytes under Simulated Ischaemia/Reperfusion. Cardiovasc. Res. 2012, 93, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Toshiyuki, T.; Tong, T.; Chin, L.N.; Roth David, M.; Brian, R.; Miho, S.; Lew Wilbur, Y.W.; Paul, C.; Kirk, H.H. Increased Cardiac Adenylyl Cyclase Expression Is Associated With Increased Survival After Myocardial Infarction. Circulation 2006, 114, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Lai, N.C.; Tang, T.; Gao, M.H.; Saito, M.; Takahashi, T.; Roth, D.M.; Hammond, H.K. Activation of Cardiac Adenylyl Cyclase Expression Increases Function of the Failing Ischemic Heart in Mice. J. Am. Coll. Cardiol. 2008, 51, 1490–1497. [Google Scholar] [CrossRef] [Green Version]
- Granrud, G.A.; Vatterott, P.J. Arrhythmias and Acute Myocardial Infarction. Postgrad. Med. 1991, 90, 85–88. [Google Scholar] [CrossRef]
- Mattick, P.; Parrington, J.; Odia, E.; Simpson, A.; Collins, T.; Terrar, D. Ca2+-Stimulated Adenylyl Cyclase Isoform AC1 Is Preferentially Expressed in Guinea-Pig Sino-Atrial Node Cells and Modulates the I(f) Pacemaker Current. J. Physiol. 2007, 582, 1195–1203. [Google Scholar] [CrossRef]
- Boink, G.J.J.; Nearing, B.D.; Shlapakova, I.N.; Duan, L.; Kryukova, Y.; Bobkov, Y.; Tan, H.L.; Cohen, I.S.; Danilo, P.; Robinson, R.B.; et al. Ca 2+ -Stimulated Adenylyl Cyclase AC1 Generates Efficient Biological Pacing as Single Gene Therapy and in Combination With HCN2. Circulation 2012, 126, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, R.S.; Naugle, J.E.; Hase, M.; Gregorian, C.; Swaney, J.S.; Insel, P.A.; Brunton, L.L.; Meszaros, J.G. Angiotensin II Enhances Adenylyl Cyclase Signaling via Ca2+/Calmodulin Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts. J. Biol. Chem. 2003, 278, 24461–24468. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hof, T.; Baldwin, T.A.; Chen, L.; Kass, R.S.; Dessauer, C.W. Regulation of IKs Potassium Current by Isoproterenol in Adult Cardiomyocytes Requires Type 9 Adenylyl Cyclase. Cells 2019, 8, 981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Chen, L.; Kass, R.S.; Dessauer, C.W. The A-Kinase Anchoring Protein Yotiao Facilitates Complex Formation between Adenylyl Cyclase Type 9 and the IKs Potassium Channel in Heart. J. Biol. Chem. 2012, 287, 29815–29824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, J.; Ren, X.; Wang, X.; Zhang, P.; Jones, W.K.; Molkentin, J.D.; Fan, G.-C.; Kranias, E.G. Blockade of Hsp20 Phosphorylation Exacerbates Cardiac Ischemia/Reperfusion Injury by Suppressed Autophagy and Increased Cell Death. Circ. Res. 2009, 105, 1223–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinaldi, L.; Pozdniakova, S.; Jayarajan, V.; Troidl, C.; Abdallah, Y.; Aslam, M.; Ladilov, Y. Protective Role of Soluble Adenylyl Cyclase against Reperfusion-Induced Injury of Cardiac Cells. Biochim. Et Biophys. Acta Mol. Basis Dis. 2019, 1865, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Acin-Perez, R.; Salazar, E.; Kamenetsky, M.; Buck, J.; Levin, L.R.; Manfredi, G. Cyclic AMP Produced inside Mitochondria Regulates Oxidative Phosphorylation. Cell Metab. 2009, 9, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newlon, M.G.; Roy, M.; Morikis, D.; Carr, D.W.; Westphal, R.; Scott, J.D.; Jennings, P.A. A Novel Mechanism of PKA Anchoring Revealed by Solution Structures of Anchoring Complexes. EMBO J. 2001, 20, 1651–1662. [Google Scholar] [CrossRef] [Green Version]
- Haushalter, K.J.; Casteel, D.E.; Raffeiner, A.; Stefan, E.; Patel, H.H.; Taylor, S.S. Phosphorylation of Protein Kinase A (PKA) Regulatory Subunit RIα by Protein Kinase G (PKG) Primes PKA for Catalytic Activity in Cells. J. Biol. Chem. 2018, 293, 4411–4421. [Google Scholar] [CrossRef] [Green Version]
- Kopperud, R.; Christensen, A.E.; Kjarland, E.; Viste, K.; Kleivdal, H.; Doskeland, S.O. Formation of Inactive CAMP-Saturated Holoenzyme of CAMP-Dependent Protein Kinase under Physiological Conditions. J. Biol. Chem. 2002, 277, 13443–13448. [Google Scholar] [CrossRef] [Green Version]
- Francis, S.H.; Corbin, J.D. Structure and Function of Cyclic Nucleotide-Dependent Protein Kinases. Annu Rev. Physiol. 1994, 56, 237–272. [Google Scholar] [CrossRef]
- Smith, F.D.; Esseltine, J.L.; Nygren, P.J.; Veesler, D.; Byrne, D.P.; Vonderach, M.; Strashnov, I.; Eyers, C.E.; Eyers, P.A.; Langeberg, L.K.; et al. Local Protein Kinase A Action Proceeds through Intact Holoenzymes. Science 2017, 356, 1288–1293. [Google Scholar] [CrossRef] [Green Version]
- Reimann, E.M.; Walsh, D.A.; Krebs, E.G. Purification and Properties of Rabbit Skeletal Muscle Adenosine 3’,5’-Monophosphate-Dependent Protein Kinases. J. Biol. Chem. 1971, 246, 1986–1995. [Google Scholar] [CrossRef]
- Corbin, J.D.; Keely, S.L.; Park, C.R. The Distribution and Dissociation of Cyclic Adenosine 3’:5’-Monophosphate-Dependent Protein Kinases in Adipose, Cardiac, and Other Tissues. J. Biol. Chem. 1975, 250, 218–225. [Google Scholar] [CrossRef]
- Uhler, M.D.; Chrivia, J.C.; McKnight, G.S. Evidence for a Second Isoform of the Catalytic Subunit of CAMP-Dependent Protein Kinase. J. Biol. Chem. 1986, 261, 15360–15363. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.E.; Zhang, X.; Li, Y.; Chen, B.; Liu, C.; Ai, X.; Zhang, X.; Tian, Y.; Zhang, C.; et al. Cardiomyocyte PKA Ablation Enhances Basal Contractility While Eliminates Cardiac β-Adrenergic Response Without Adverse Effects on the Heart. Circ. Res. 2019, 124, 1760–1777. [Google Scholar] [CrossRef]
- Feng, G.; Yan, Z.; Li, C.; Hou, Y. MicroRNA-208a in an Early Stage Myocardial Infarction Rat Model and the Effect on CAMP-PKA Signaling Pathway. Mol. Med. Rep. 2016, 14, 1631–1635. [Google Scholar] [CrossRef] [Green Version]
- Haushalter, K.J.; Schilling, J.M.; Song, Y.; Sastri, M.; Perkins, G.A.; Strack, S.; Taylor, S.S.; Patel, H.H. Cardiac Ischemia-Reperfusion Injury Induces ROS-Dependent Loss of PKA Regulatory Subunit RIα. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1231–H1242. [Google Scholar] [CrossRef]
- Han, Y.S.; Arroyo, J.; Ogut, O. Human Heart Failure Is Accompanied by Altered Protein Kinase A Subunit Expression and Post-Translational State. Arch. Biochem. Biophys. 2013, 538, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Kronenbitter, A.; Funk, F.; Hackert, K.; Gorreßen, S.; Glaser, D.; Boknik, P.; Poschmann, G.; Stühler, K.; Isić, M.; Krüger, M.; et al. Impaired Ca2+ Cycling of Nonischemic Myocytes Contributes to Sarcomere Dysfunction Early after Myocardial Infarction. J. Mol. Cell. Cardiol. 2018, 119, 28–39. [Google Scholar] [CrossRef]
- Piddo, A.M.; Sánchez, M.I.; Sapag-Hagar, M.; Corbalán, R.; Foncea, R.; Ebensperger, R.; Godoy, I.; Meléndez, J.; Jalil, J.E.; Lavandero, S. Cyclic AMP-Dependent Protein Kinase and Mechanical Heart Function in Ventricular Hypertrophy Induced by Pressure Overload or Secondary to Myocardial Infarction. J. Mol. Cell. Cardiol. 1996, 28, 1073–1083. [Google Scholar] [CrossRef]
- Li, X.-D.; Cheng, Y.-T.; Yang, Y.-J.; Meng, X.-M.; Zhao, J.-L.; Zhang, H.-T.; Wu, Y.-J.; You, S.-J.; Wu, Y.-L. PKA-Mediated ENOS Phosphorylation in the Protection of Ischemic Preconditioning against No-Reflow. Microvasc. Res. 2012, 84, 44–54. [Google Scholar] [CrossRef]
- Sanada, S.; Asanuma, H.; Tsukamoto, O.; Minamino, T.; Node, K.; Takashima, S.; Fukushima, T.; Ogai, A.; Shinozaki, Y.; Fujita, M.; et al. Protein Kinase A as Another Mediator of Ischemic Preconditioning Independent of Protein Kinase C. Circulation 2004, 110, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.-M.; Lin, S.-Z.; Chang, N.-C. Both PKA and Epac Pathways Mediate N-Acetylcysteine-Induced Connexin43 Preservation in Rats with Myocardial Infarction. PLoS ONE 2013, 8, e71878. [Google Scholar] [CrossRef] [Green Version]
- Nishida, H.; Sato, T.; Miyazaki, M.; Nakaya, H. Infarct Size Limitation by Adrenomedullin: Protein Kinase A but Not PI3-Kinase Is Linked to Mitochondrial KCa Channels. Cardiovasc. Res. 2008, 77, 398–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Keyes, K.T.; Zhang, C.; Perez-Polo, J.R.; Lin, Y.; Birnbaum, Y. The Myocardial Infarct Size-Limiting Effect of Sitagliptin Is PKA-Dependent, Whereas the Protective Effect of Pioglitazone Is Partially Dependent on PKA. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1454–H1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yang, Y.; Geng, Y.; Zhao, J.; Zhang, H.; Cheng, Y.; Wu, Y. Phosphorylation of Endothelial NOS Contributes to Simvastatin Protection against Myocardial No-Reflow and Infarction in Reperfused Swine Hearts: Partially via the PKA Signaling Pathway. Acta Pharmacol. Sin. 2012, 33, 879–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulucan, C.; Wang, X.; Baljinnyam, E.; Bai, Y.; Okumura, S.; Sato, M.; Minamisawa, S.; Hirotani, S.; Ishikawa, Y. Developmental Changes in Gene Expression of Epac and Its Upregulation in Myocardial Hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1662–H1672. [Google Scholar] [CrossRef] [Green Version]
- Bos, J.L. Epac Proteins: Multi-Purpose CAMP Targets. Trends Biochem. Sci. 2006, 31, 680–686. [Google Scholar] [CrossRef]
- Ruiz-Hurtado, G.; Morel, E.; Domínguez-Rodríguez, A.; Llach, A.; Lezoualc’h, F.; Benitah, J.-P.; Gomez, A.M. Epac in Cardiac Calcium Signaling. J. Mol. Cell Cardiol. 2013, 58, 162–171. [Google Scholar] [CrossRef]
- Pereira, L.; Rehmann, H.; Lao, D.H.; Erickson, J.R.; Bossuyt, J.; Chen, J.; Bers, D.M. Novel Epac Fluorescent Ligand Reveals Distinct Epac1 vs. Epac2 Distribution and Function in Cardiomyocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 3991–3996. [Google Scholar] [CrossRef] [Green Version]
- Fujita, T.; Umemura, M.; Yokoyama, U.; Okumura, S.; Ishikawa, Y. The Role of Epac in the Heart. Cell. Mol. Life Sci. 2017, 74, 591–606. [Google Scholar] [CrossRef]
- Kryzhanovsky, S.A.; Nikiforova, T.D.; Durnev, A.D. Epac Proteins and Their Role in the Physiological and Pathological Processes in the Cardiovascular System. Part 1: The Role of Epac Proteins in the Physiological and Pathological Processes of the Vasculature. Hum. Physiol. 2020, 46, 200–215. [Google Scholar] [CrossRef]
- Yokoyama, U.; Patel, H.H.; Lai, N.C.; Aroonsakool, N.; Roth, D.M.; Insel, P.A. The Cyclic AMP Effector Epac Integrates Pro- and Anti-Fibrotic Signals. Proc. Natl. Acad. Sci. USA 2008, 105, 6386–6391. [Google Scholar] [CrossRef] [Green Version]
- Surinkaew, S.; Aflaki, M.; Takawale, A.; Chen, Y.; Qi, X.-Y.; Gillis, M.-A.; Shi, Y.-F.; Tardif, J.-C.; Chattipakorn, N.; Nattel, S. Exchange Protein Activated by Cyclic-Adenosine Monophosphate (Epac) Regulates Atrial Fibroblast Function and Controls Cardiac Remodelling. Cardiovasc Res. 2019, 115, 94–106. [Google Scholar] [CrossRef]
- Khan, I.; Ali, A.; Akhter, M.A.; Naeem, N.; Chotani, M.A.; Iqbal, H.; Kabir, N.; Atiq, M.; Salim, A. Epac-Rap1-Activated Mesenchymal Stem Cells Improve Cardiac Function in Rat Model of Myocardial Infarction. Cardiovasc 2017, 35. [Google Scholar] [CrossRef]
- Biel, M. Cyclic Nucleotide-Regulated Cation Channels. J. Biol. Chem. 2009, 284, 9017–9021. [Google Scholar] [CrossRef] [Green Version]
- Scicchitano, P.; Carbonara, S.; Ricci, G.; Mandurino, C.; Locorotondo, M.; Bulzis, G.; Gesualdo, M.; Zito, A.; Carbonara, R.; Dentamaro, I.; et al. HCN Channels and Heart Rate. Molecules 2012, 17, 4225–4235. [Google Scholar] [CrossRef]
- Baruscotti, M.; Difrancesco, D. Pacemaker Channels. Ann. N.Y. Acad. Sci. 2004, 1015, 111–121. [Google Scholar] [CrossRef]
- Xia, S.; Wang, Y.; Zhang, Y.; Deng, S.-B.; Du, J.-L.; Wang, X.-C.; She, Q. Dynamic Changes in HCN2, HCN4, KCNE1, and KCNE2 Expression in Ventricular Cells from Acute Myocardial Infarction Rat Hearts. Biochem. Biophys. Res. Commun. 2010, 395, 330–335. [Google Scholar] [CrossRef]
- Mackiewicz, U.; Gerges, J.Y.; Chu, S.; Duda, M.; Dobrzynski, H.; Lewartowski, B.; Mączewski, M. Ivabradine Protects Against Ventricular Arrhythmias in Acute Myocardial Infarction in the Rat. J. Cell. Physiol. 2014, 229, 813–823. [Google Scholar] [CrossRef]
- Song, T.; Yang, J.; Yao, Y.; Li, H.; Chen, Y.; Zhang, J.; Huang, C. Spironolactone Diminishes Spontaneous Ventricular Premature Beats by Reducing HCN4 Protein Expression in Rats with Myocardial Infarction. Mol. Med. Rep. 2011, 4, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Brand, T. POPDC Proteins and Cardiac Function. Biochem. Soc. Trans. 2019, 47, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Froese, A.; Breher, S.S.; Waldeyer, C.; Schindler, R.F.R.; Nikolaev, V.O.; Rinné, S.; Wischmeyer, E.; Schlueter, J.; Becher, J.; Simrick, S.; et al. Popeye Domain Containing Proteins Are Essential for Stress-Mediated Modulation of Cardiac Pacemaking in Mice. J. Clin. Invest. 2012, 122, 1119–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindler, R.F.R.; Scotton, C.; Zhang, J.; Passarelli, C.; Ortiz-Bonnin, B.; Simrick, S.; Schwerte, T.; Poon, K.-L.; Fang, M.; Rinné, S.; et al. POPDC1(S201F) Causes Muscular Dystrophy and Arrhythmia by Affecting Protein Trafficking. J. Clin. Investig. 2016, 126, 239–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchmaier, B.C.; Poon, K.L.; Schwerte, T.; Huisken, J.; Winkler, C.; Jungblut, B.; Stainier, D.Y.; Brand, T. The Popeye Domain Containing 2 (Popdc2) Gene in Zebrafish Is Required for Heart and Skeletal Muscle Development. Dev. Biol. 2012, 363, 438–450. [Google Scholar] [CrossRef] [Green Version]
- De Ridder, W.; Nelson, I.; Asselbergh, B.; De Paepe, B.; Beuvin, M.; Ben Yaou, R.; Masson, C.; Boland, A.; Deleuze, J.-F.; Maisonobe, T.; et al. Muscular Dystrophy with Arrhythmia Caused by Loss-of-Function Mutations in BVES. Neurol. Genet. 2019, 5, e321. [Google Scholar] [CrossRef] [Green Version]
- Meinke, P.; Kerr, A.R.W.; Czapiewski, R.; de Las Heras, J.I.; Dixon, C.R.; Harris, E.; Kölbel, H.; Muntoni, F.; Schara, U.; Straub, V.; et al. A Multistage Sequencing Strategy Pinpoints Novel Candidate Alleles for Emery-Dreifuss Muscular Dystrophy and Supports Gene Misregulation as Its Pathomechanism. EBioMedicine 2020, 51, 102587. [Google Scholar] [CrossRef] [Green Version]
- Alcalay, Y.; Hochhauser, E.; Kliminski, V.; Dick, J.; Zahalka, M.A.; Parnes, D.; Schlesinger, H.; Abassi, Z.; Shainberg, A.; Schindler, R.F.R.; et al. Popeye Domain Containing 1 (Popdc1/Bves) Is a Caveolae-Associated Protein Involved in Ischemia Tolerance. PLoS ONE 2013, 8, e71100. [Google Scholar] [CrossRef] [Green Version]
- Marin, W. A-Kinase Anchoring Protein 1 (AKAP1) and Its Role in Some Cardiovascular Diseases. J. Mol. Cell. Cardiol. 2020, 138, 99–109. [Google Scholar] [CrossRef]
- Carr, D.W.; Stofko-Hahn, R.E.; Fraser, I.D.; Bishop, S.M.; Acott, T.S.; Brennan, R.G.; Scott, J.D. Interaction of the Regulatory Subunit (RII) of CAMP-Dependent Protein Kinase with RII-Anchoring Proteins Occurs through an Amphipathic Helix Binding Motif. J. Biol. Chem. 1991, 266, 14188–14192. [Google Scholar] [CrossRef]
- Calejo, A.I.; Taskén, K. Targeting Protein-Protein Interactions in Complexes Organized by A Kinase Anchoring Proteins. Front. Pharmacol. 2015, 6, 192. [Google Scholar] [CrossRef] [Green Version]
- Dodge-Kafka, K.L.; Soughayer, J.; Pare, G.C.; Carlisle Michel, J.J.; Langeberg, L.K.; Kapiloff, M.S.; Scott, J.D. The Protein Kinase A Anchoring Protein MAKAP Coordinates Two Integrated CAMP Effector Pathways. Nature 2005, 437, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Taskén, K.; Aandahl, E.M. Localized Effects of CAMP Mediated by Distinct Routes of Protein Kinase A. Physiol. Rev. 2004, 84, 137–167. [Google Scholar] [CrossRef] [Green Version]
- Esseltine, J.L.; Scott, J.D. AKAP Signaling Complexes: Pointing towards the next Generation of Therapeutic Targets? Trends Pharm. Sci 2013, 34, 648–655. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.D.; Dessauer, C.W.; Taskén, K. Creating Order from Chaos: Cellular Regulation by Kinase Anchoring. Annu Rev. Pharmacol. Toxicol. 2013, 53, 187–210. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.J.; Durick, K.; Weiner, J.A.; Chun, J.; Taylor, S.S. Identification of a Novel Protein Kinase A Anchoring Protein That Binds Both Type I and Type II Regulatory Subunits. J. Biol. Chem. 1997, 272, 8057–8064. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.J.; Durick, K.; Weiner, J.A.; Chun, J.; Taylor, S.S. D-AKAP2, a Novel Protein Kinase A Anchoring Protein with a Putative RGS Domain. Proc. Natl. Acad. Sci. USA 1997, 94, 11184–11189. [Google Scholar] [CrossRef] [Green Version]
- Means, C.K.; Lygren, B.; Langeberg, L.K.; Jain, A.; Dixon, R.E.; Vega, A.L.; Gold, M.G.; Petrosyan, S.; Taylor, S.S.; Murphy, A.N.; et al. An Entirely Specific Type I A-Kinase Anchoring Protein That Can Sequester Two Molecules of Protein Kinase A at Mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, E1227–E1235. [Google Scholar] [CrossRef] [Green Version]
- Burgers, P.P.; Ma, Y.; Margarucci, L.; Mackey, M.; van der Heyden, M.A.G.; Ellisman, M.; Scholten, A.; Taylor, S.S.; Heck, A.J.R. A Small Novel A-Kinase Anchoring Protein (AKAP) That Localizes Specifically Protein Kinase A-Regulatory Subunit I (PKA-RI) to the Plasma Membrane. J. Biol. Chem. 2012, 287, 43789–43797. [Google Scholar] [CrossRef] [Green Version]
- Perrino, C.; Feliciello, A.; Schiattarella, G.G.; Esposito, G.; Guerriero, R.; Zaccaro, L.; Del Gatto, A.; Saviano, M.; Garbi, C.; Carangi, R.; et al. AKAP121 Downregulation Impairs Protective CAMP Signals, Promotes Mitochondrial Dysfunction, and Increases Oxidative Stress. Cardiovasc. Res. 2010, 88, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, J.L.; Dell’Acqua, M.L. AKAP Signaling Complexes in Regulation of Excitatory Synaptic Plasticity. Neuroscientist 2011, 17, 321–336. [Google Scholar] [CrossRef] [Green Version]
- Efendiev, R.; Samelson, B.K.; Nguyen, B.T.; Phatarpekar, P.V.; Baameur, F.; Scott, J.D.; Dessauer, C.W. AKAP79 Interacts with Multiple Adenylyl Cyclase (AC) Isoforms and Scaffolds AC5 and -6 to Alpha-Amino-3-Hydroxyl-5-Methyl-4-Isoxazole-Propionate (AMPA) Receptors. J. Biol. Chem. 2010, 285, 14450–14458. [Google Scholar] [CrossRef] [Green Version]
- Nichols, C.B.; Rossow, C.F.; Navedo, M.F.; Westenbroek, R.E.; Catterall, W.A.; Santana, L.F.; McKnight, G.S. Sympathetic Stimulation of Adult Cardiomyocytes Requires Association of AKAP5 with a Subpopulation of L-Type Calcium Channels. Circ. Res. 2010, 107, 747–756. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Drum, B.M.; Chen, Y.; Yin, H.; Guo, X.; Luckey, S.W.; Gilbert, M.L.; McKnight, G.S.; Scott, J.D.; et al. Loss of AKAP150 Promotes Pathological Remodelling and Heart Failure Propensity by Disrupting Calcium Cycling and Contractile Reserve. Cardiovasc. Res. 2017, 113, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Nieves-Cintrón, M.; Hirenallur-Shanthappa, D.; Nygren, P.J.; Hinke, S.A.; Dell’Acqua, M.L.; Langeberg, L.K.; Navedo, M.; Santana, L.F.; Scott, J.D. AKAP150 Participates in Calcineurin/NFAT Activation during the down-Regulation of Voltage-Gated K+ Currents in Ventricular Myocytes Following Myocardial Infarction. Cell. Signal. 2016, 28, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Kapiloff, M.S.; Schillace, R.V.; Westphal, A.M.; Scott, J.D. MAKAP: An A-Kinase Anchoring Protein Targeted to the Nuclear Membrane of Differentiated Myocytes. J. Cell Sci. 1999, 112 (Pt 16), 2725–2736. [Google Scholar]
- Passariello, C.L.; Li, J.; Dodge-Kafka, K.; Kapiloff, M.S. MAKAP-a Master Scaffold for Cardiac Remodeling. J. Cardiovasc. Pharm. 2015, 65, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Kapiloff, M.S.; Piggott, L.A.; Sadana, R.; Li, J.; Heredia, L.A.; Henson, E.; Efendiev, R.; Dessauer, C.W. An Adenylyl Cyclase-MAKAPbeta Signaling Complex Regulates CAMP Levels in Cardiac Myocytes. J. Biol. Chem. 2009, 284, 23540–23546. [Google Scholar] [CrossRef] [Green Version]
- Jansen, S.; Jorgensen, J.; Caplehorn, J.; Hunt, D. Preoperative Ultrasound to Predict Conversion in Laparoscopic Cholecystectomy. Surg. Laparosc. Endosc. 1997, 7, 121–123. [Google Scholar] [CrossRef]
- Lee, S.H.; Wolf, P.L.; Escudero, R.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Early Expression of Angiogenesis Factors in Acute Myocardial Ischemia and Infarction. N. Engl. J. Med. 2000, 342, 626–633. [Google Scholar] [CrossRef]
- Hausken, Z.E.; Dell’Acqua, M.L.; Coghlan, V.M.; Scott, J.D. Mutational Analysis of the A-Kinase Anchoring Protein (AKAP)-Binding Site on RII. Classification Of Side Chain Determinants for Anchoring and Isoform Selective Association with AKAPs. J. Biol. Chem. 1996, 271, 29016–29022. [Google Scholar] [CrossRef] [Green Version]
- Meacci, E.; Taira, M.; Moos, M.; Smith, C.J.; Movsesian, M.A.; Degerman, E.; Belfrage, P.; Manganiello, V. Molecular Cloning and Expression of Human Myocardial CGMP-Inhibited CAMP Phosphodiesterase. Proc. Natl. Acad. Sci. USA 1992, 89, 3721–3725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Yao, Z.; Zhao, W.; Zhang, Y.; Yao, C.; Tong, C. MiR-21 Enhances the Protective Effect of Loperamide on Rat Cardiomyocytes against Hypoxia/Reoxygenation, Reactive Oxygen Species Production and Apoptosis via Regulating Akap8 and Bard1 Expression. Exp. Med. 2019, 17, 1312–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammerer, S.; Burns-Hamuro, L.L.; Ma, Y.; Hamon, S.C.; Canaves, J.M.; Shi, M.M.; Nelson, M.R.; Sing, C.F.; Cantor, C.R.; Taylor, S.S.; et al. Amino Acid Variant in the Kinase Binding Domain of Dual-Specific A Kinase-Anchoring Protein 2: A Disease Susceptibility Polymorphism. Proc. Natl. Acad. Sci. USA 2003, 100, 4066–4071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishihama, K.; Yamada, Y.; Matsuo, H.; Segawa, T.; Watanabe, S.; Kato, K.; Yajima, K.; Hibino, T.; Yokoi, K.; Ichihara, S.; et al. Association of Gene Polymorphisms with Myocardial Infarction in Individuals with or without Conventional Coronary Risk Factors. Int. J. Mol. Med. 2007, 19, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malbon, C.C.; Tao, J.; Shumay, E.; Wang, H.-Y. AKAP (A-Kinase Anchoring Protein) Domains: Beads of Structure-Function on the Necklace of G-Protein Signalling. Biochem. Soc. Trans. 2004, 32, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Shih, M.; Lin, F.; Scott, J.D.; Wang, H.Y.; Malbon, C.C. Dynamic Complexes of Beta2-Adrenergic Receptors with Protein Kinases and Phosphatases and the Role of Gravin. J. Biol. Chem. 1999, 274, 1588–1595. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Wang, H.Y.; Malbon, C.C. Gravin-Mediated Formation of Signaling Complexes in Beta 2-Adrenergic Receptor Desensitization and Resensitization. J. Biol. Chem. 2000, 275, 19025–19034. [Google Scholar] [CrossRef] [Green Version]
- Guillory, A.N.; Yin, X.; Wijaya, C.S.; Diaz Diaz, A.C.; Rababa’h, A.; Singh, S.; Atrooz, F.; Sadayappan, S.; McConnell, B.K. Enhanced Cardiac Function in Gravin Mutant Mice Involves Alterations in the β-Adrenergic Receptor Signaling Cascade. PLoS ONE 2013, 8, e74784. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yu, Q.-H.; Chu, Y.; Wu, W.-M.; Song, J.-X.; Zhu, X.-B.; Wang, Q. Blockage of AKAP12 Accelerates Angiotensin II (Ang II)-Induced Cardiac Injury in Mice by Regulating the Transforming Growth Factor Β1 (TGF-Β1) Pathway. Biochem. Biophys. Res. Commun. 2018, 499, 128–135. [Google Scholar] [CrossRef]
- Diviani, D.; Osman, H.; Delaunay, M.; Kaiser, S. The Role of A-Kinase Anchoring Proteins in Cardiac Oxidative Stress. Biochem. Soc. Trans. 2019, 47, 1341–1353. [Google Scholar] [CrossRef]
- Kim, H.; Scimia, M.C.; Wilkinson, D.; Trelles, R.D.; Wood, M.R.; Bowtell, D.; Dillin, A.; Mercola, M.; Ronai, Z.A. Fine-Tuning of Drp1/Fis1 Availability by AKAP121/Siah2 Regulates Mitochondrial Adaptation to Hypoxia. Mol. Cell. 2011, 44, 532–544. [Google Scholar] [CrossRef] [Green Version]
- Schiattarella, G.G.; Cattaneo, F.; Pironti, G.; Magliulo, F.; Carotenuto, G.; Pirozzi, M.; Polishchuk, R.; Borzacchiello, D.; Paolillo, R.; Oliveti, M.; et al. Correction: Akap1 Deficiency Promotes Mitochondrial Aberrations and Exacerbates Cardiac Injury Following Permanent Coronary Ligation via Enhanced Mitophagy and Apoptosis. PLoS ONE 2016, 11, e0158934. [Google Scholar] [CrossRef] [Green Version]
- Bauman, A.L.; Soughayer, J.; Nguyen, B.T.; Willoughby, D.; Carnegie, G.K.; Wong, W.; Hoshi, N.; Langeberg, L.K.; Cooper, D.M.F.; Dessauer, C.W.; et al. Dynamic Regulation of CAMP Synthesis through Anchored PKA-Adenylyl Cyclase V/VI Complexes. Mol. Cell. 2006, 23, 925–931. [Google Scholar] [CrossRef] [Green Version]
- Ruehr, M.L.; Russell, M.A.; Bond, M. A-Kinase Anchoring Protein Targeting of Protein Kinase A in the Heart. J. Mol. Cell. Cardiol. 2004, 37, 653–665. [Google Scholar] [CrossRef]
- Collas, P.; Le Guellec, K.; Taskén, K. The A-Kinase-Anchoring Protein AKAP95 Is a Multivalent Protein with a Key Role in Chromatin Condensation at Mitosis. J. Cell. Biol. 1999, 147, 1167–1180. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, B.; Zhou, Q.; Wang, Y.; Liu, X.; Liu, Z.; Zhan, Z. MicroRNA-21 Prevents Excessive Inflammation and Cardiac Dysfunction after Myocardial Infarction through Targeting KBTBD7. Cell. Death Dis. 2018, 9, 769. [Google Scholar] [CrossRef] [Green Version]
- Sarma, G.N.; Moody, I.S.; Ilouz, R.; Phan, R.H.; Sankaran, B.; Hall, R.A.; Taylor, S.S. D-AKAP2:PKA RII:PDZK1 Ternary Complex Structure: Insights from the Nucleation of a Polyvalent Scaffold. Protein. Sci. 2015, 24, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Tingley, W.G.; Pawlikowska, L.; Zaroff, J.G.; Kim, T.; Nguyen, T.; Young, S.G.; Vranizan, K.; Kwok, P.-Y.; Whooley, M.A.; Conklin, B.R. Gene-Trapped Mouse Embryonic Stem Cell-Derived Cardiac Myocytes and Human Genetics Implicate AKAP10 in Heart Rhythm Regulation. Proc. Natl. Acad. Sci. USA 2007, 104, 8461–8466. [Google Scholar] [CrossRef] [Green Version]
- Fraser, I.D.; Tavalin, S.J.; Lester, L.B.; Langeberg, L.K.; Westphal, A.M.; Dean, R.A.; Marrion, N.V.; Scott, J.D. A Novel Lipid-Anchored A-Kinase Anchoring Protein Facilitates CAMP-Responsive Membrane Events. EMBO J. 1998, 17, 2261–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulme, J.T.; Lin, T.W.-C.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Beta-Adrenergic Regulation Requires Direct Anchoring of PKA to Cardiac CaV1.2 Channels via a Leucine Zipper Interaction with A Kinase-Anchoring Protein 15. Proc. Natl. Acad. Sci. USA 2003, 100, 13093–13098. [Google Scholar] [CrossRef] [Green Version]
- Hulme, J.T.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Phosphorylation of Serine 1928 in the Distal C-Terminal Domain of Cardiac CaV1.2 Channels during Beta1-Adrenergic Regulation. Proc. Natl. Acad. Sci. USA 2006, 103, 16574–16579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bünemann, M.; Gerhardstein, B.L.; Gao, T.; Hosey, M.M. Functional Regulation of L-Type Calcium Channels via Protein Kinase A-Mediated Phosphorylation of the Beta(2) Subunit. J. Biol. Chem. 1999, 274, 33851–33854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lygren, B.; Carlson, C.R.; Santamaria, K.; Lissandron, V.; McSorley, T.; Litzenberg, J.; Lorenz, D.; Wiesner, B.; Rosenthal, W.; Zaccolo, M.; et al. AKAP Complex Regulates Ca2+ Re-Uptake into Heart Sarcoplasmic Reticulum. EMBO Rep. 2007, 8, 1061–1067. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Shen, W.; Vandeput, F.; Szabo-Fresnais, N.; Krall, J.; Degerman, E.; Goetz, F.; Klussmann, E.; Movsesian, M.; Manganiello, V. Regulation of Sarcoplasmic Reticulum Ca2+ ATPase 2 (SERCA2) Activity by Phosphodiesterase 3A (PDE3A) in Human Myocardium: Phosphorylation-Dependent Interaction of PDE3A1 with SERCA2. J. Biol. Chem. 2015, 290, 6763–6776. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Redden, J.M.; Kapiloff, M.S.; Dodge-Kafka, K.L. The Large Isoforms of A-Kinase Anchoring Protein 18 Mediate the Phosphorylation of Inhibitor-1 by Protein Kinase A and the Inhibition of Protein Phosphatase 1 Activity. Mol. Pharm. 2011, 79, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Marx, S.O.; Kurokawa, J.; Reiken, S.; Motoike, H.; D’Armiento, J.; Marks, A.R.; Kass, R.S. Requirement of a Macromolecular Signaling Complex for Beta Adrenergic Receptor Modulation of the KCNQ1-KCNE1 Potassium Channel. Science 2002, 295, 496–499. [Google Scholar] [CrossRef] [Green Version]
- Ponomarenko, M.P.; Beniukh, D.N.; Orlov, I.L.; Kolchanov, N.A. [Precise recognition method of structure-function determinants of protein molecules]. Biofizika 1991, 36, 943–956. [Google Scholar]
- Diviani, D.; Soderling, J.; Scott, J.D. AKAP-Lbc Anchors Protein Kinase A and Nucleates Galpha 12-Selective Rho-Mediated Stress Fiber Formation. J. Biol. Chem. 2001, 276, 44247–44257. [Google Scholar] [CrossRef] [Green Version]
- Cavin, S.; Maric, D.; Diviani, D. A-Kinase Anchoring Protein-Lbc Promotes pro-Fibrotic Signaling in Cardiac Fibroblasts. Biochim Biophys Acta 2014, 1843, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Diviani, D.; Osman, H.; Reggi, E. A-Kinase Anchoring Protein-Lbc: A Molecular Scaffold Involved in Cardiac Protection. J. Cardiovasc. Dev. Dis. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Abdul Azeez, K.R.; Knapp, S.; Fernandes, J.M.P.; Klussmann, E.; Elkins, J.M. The Crystal Structure of the RhoA-AKAP-Lbc DH-PH Domain Complex. Biochem. J. 2014, 464, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Appert-Collin, A.; Cotecchia, S.; Nenniger-Tosato, M.; Pedrazzini, T.; Diviani, D. The A-Kinase Anchoring Protein (AKAP)-Lbc-Signaling Complex Mediates Alpha1 Adrenergic Receptor-Induced Cardiomyocyte Hypertrophy. Proc. Natl. Acad. Sci. USA 2007, 104, 10140–10145. [Google Scholar] [CrossRef] [Green Version]
- Cariolato, L.; Cavin, S.; Diviani, D. A-Kinase Anchoring Protein (AKAP)-Lbc Anchors a PKN-Based Signaling Complex Involved in A1-Adrenergic Receptor-Induced P38 Activation. J. Biol. Chem. 2011, 286, 7925–7937. [Google Scholar] [CrossRef] [Green Version]
- Pérez López, I.; Cariolato, L.; Maric, D.; Gillet, L.; Abriel, H.; Diviani, D. A-Kinase Anchoring Protein Lbc Coordinates a P38 Activating Signaling Complex Controlling Compensatory Cardiac Hypertrophy. Mol. Cell. Biol. 2013, 33, 2903–2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnegie, G.K.; Smith, F.D.; McConnachie, G.; Langeberg, L.K.; Scott, J.D. AKAP-Lbc Nucleates a Protein Kinase D Activation Scaffold. Mol. Cell. 2004, 15, 889–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnegie, G.K.; Soughayer, J.; Smith, F.D.; Pedroja, B.S.; Zhang, F.; Diviani, D.; Bristow, M.R.; Kunkel, M.T.; Newton, A.C.; Langeberg, L.K.; et al. AKAP-Lbc Mobilizes a Cardiac Hypertrophy Signaling Pathway. Mol. Cell. 2008, 32, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lester, L.B.; Coghlan, V.M.; Nauert, B.; Scott, J.D. Cloning and Characterization of a Novel A-Kinase Anchoring Protein. AKAP 220, Association with Testicular Peroxisomes. J. Biol. Chem. 1996, 271, 9460–9465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dransfield, D.T.; Bradford, A.J.; Smith, J.; Martin, M.; Roy, C.; Mangeat, P.H.; Goldenring, J.R. Ezrin Is a Cyclic AMP-Dependent Protein Kinase Anchoring Protein. EMBO J. 1997, 16, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Adamik, R.; Pacheco-Rodriguez, G.; Moss, J.; Vaughan, M. Protein Kinase A-Anchoring (AKAP) Domains in Brefeldin A-Inhibited Guanine Nucleotide-Exchange Protein 2 (BIG2). Proc. Natl. Acad. Sci. USA 2003, 100, 1627–1632. [Google Scholar] [CrossRef] [Green Version]
- Russell, M.A.; Lund, L.M.; Haber, R.; McKeegan, K.; Cianciola, N.; Bond, M. The Intermediate Filament Protein, Synemin, Is an AKAP in the Heart. Arch. Biochem. Biophys. 2006, 456, 204–215. [Google Scholar] [CrossRef]
- Reynolds, J.G.; McCalmon, S.A.; Tomczyk, T.; Naya, F.J. Identification and Mapping of Protein Kinase A Binding Sites in the Costameric Protein Myospryn. Biochim. Biophys. Acta 2007, 1773, 891–902. [Google Scholar] [CrossRef] [Green Version]
- Sumandea, C.A.; Garcia-Cazarin, M.L.; Bozio, C.H.; Sievert, G.A.; Balke, C.W.; Sumandea, M.P. Cardiac Troponin T, a Sarcomeric AKAP, Tethers Protein Kinase A at the Myofilaments. J. Biol. Chem. 2011, 286, 530–541. [Google Scholar] [CrossRef] [Green Version]
- Baillie, G.S.; Scott, J.D.; Houslay, M.D. Compartmentalisation of Phosphodiesterases and Protein Kinase A: Opposites Attract. FEBS Lett. 2005, 579, 3264–3270. [Google Scholar] [CrossRef] [Green Version]
- Lugnier, C. Cyclic Nucleotide Phosphodiesterase (PDE) Superfamily: A New Target for the Development of Specific Therapeutic Agents. Pharmacol. Ther. 2006, 109, 366–398. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Lighthouse, J.K.; Mickelsen, D.M.; Wu, J.; Yao, P.; Small, E.M.; Yan, C. A Novel Role of Cyclic Nucleotide Phosphodiesterase 10A in Pathological Cardiac Remodeling and Dysfunction. Circulation 2020, 141, 217–233. [Google Scholar] [CrossRef]
- Dell’Acqua, M.L.; Scott, J.D. Protein Kinase A Anchoring. J. Biol. Chem. 1997, 272, 12881–12884. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Knight, W.E.; Yan, C. Roles of PDE1 in Pathological Cardiac Remodeling and Dysfunction. J. Cardiovasc. Dev. Dis. 2018, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Kincaid, R.L.; Stith-Coleman, I.E.; Vaughan, M. Proteolytic Activation of Calmodulin-Dependent Cyclic Nucleotide Phosphodiesterase. J. Biol. Chem. 1985, 260, 9009–9015. [Google Scholar] [CrossRef]
- Ang, K.-L.; Antoni, F.A. Reciprocal Regulation of Calcium Dependent and Calcium Independent Cyclic AMP Hydrolysis by Protein Phosphorylation. J. Neurochem. 2002, 81, 422–433. [Google Scholar] [CrossRef]
- Miller, C.L.; Cai, Y.; Oikawa, M.; Thomas, T.; Dostmann, W.R.; Zaccolo, M.; Fujiwara, K.; Yan, C. Cyclic Nucleotide Phosphodiesterase 1A: A Key Regulator of Cardiac Fibroblast Activation and Extracellular Matrix Remodeling in the Heart. Basic Res. Cardiol. 2011, 106, 1023–1039. [Google Scholar] [CrossRef] [Green Version]
- Martins, T.J.; Mumby, M.C.; Beavo, J.A. Purification and Characterization of a Cyclic GMP-Stimulated Cyclic Nucleotide Phosphodiesterase from Bovine Tissues. J. Biol. Chem. 1982, 257, 1973–1979. [Google Scholar] [CrossRef]
- Mehel, H.; Emons, J.; Vettel, C.; Wittköpper, K.; Seppelt, D.; Dewenter, M.; Lutz, S.; Sossalla, S.; Maier, L.S.; Lechêne, P.; et al. Phosphodiesterase-2 Is up-Regulated in Human Failing Hearts and Blunts β-Adrenergic Responses in Cardiomyocytes. J. Am. Coll. Cardiol. 2013, 62, 1596–1606. [Google Scholar] [CrossRef] [Green Version]
- Vettel, C.; Lämmle, S.; Ewens, S.; Cervirgen, C.; Emons, J.; Ongherth, A.; Dewenter, M.; Lindner, D.; Westermann, D.; Nikolaev, V.O.; et al. PDE2-Mediated CAMP Hydrolysis Accelerates Cardiac Fibroblast to Myofibroblast Conversion and Is Antagonized by Exogenous Activation of CGMP Signaling Pathways. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1246–H1252. [Google Scholar] [CrossRef]
- Monterisi, S.; Lobo, M.J.; Livie, C.; Castle, J.C.; Weinberger, M.; Baillie, G.; Surdo, N.C.; Musheshe, N.; Stangherlin, A.; Gottlieb, E.; et al. PDE2A2 Regulates Mitochondria Morphology and Apoptotic Cell Death via Local Modulation of CAMP/PKA Signalling. eLife 2017, 6, e21374. [Google Scholar] [CrossRef]
- Vettel, C.; Lindner, M.; Dewenter, M.; Lorenz, K.; Schanbacher, C.; Riedel, M.; Lämmle, S.; Meinecke, S.; Mason, F.E.; Sossalla, S.; et al. Phosphodiesterase 2 Protects Against Catecholamine-Induced Arrhythmia and Preserves Contractile Function After Myocardial Infarction. Circ. Res. 2017, 120, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Mongillo, M.; Tocchetti, C.G.; Terrin, A.; Lissandron, V.; Cheung, Y.-F.; Dostmann, W.R.; Pozzan, T.; Kass, D.A.; Paolocci, N.; Houslay, M.D.; et al. Compartmentalized Phosphodiesterase-2 Activity Blunts β-Adrenergic Cardiac Inotropy via an NO/CGMP-Dependent Pathway. Circ. Res. 2006, 98, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Schobesberger, S.; Wright, P.T.; Poulet, C.; Sanchez Alonso Mardones, J.L.; Mansfield, C.; Friebe, A.; Harding, S.E.; Balligand, J.-L.; Nikolaev, V.O.; Gorelik, J. Β3-Adrenoceptor Redistribution Impairs NO/CGMP/PDE2 Signalling in Failing Cardiomyocytes. eLife 2020, 9, e52221. [Google Scholar] [CrossRef]
- Miki, T.; Taira, M.; Hockman, S.; Shimada, F.; Lieman, J.; Napolitano, M.; Ward, D.; Taira, M.; Makino, H.; Manganiello, V.C. Characterization of the CDNA and Gene Encoding Human PDE3B, the CGIP1 Isoform of the Human Cyclic GMP-Inhibited Cyclic Nucleotide Phosphodiesterase Family. Genomics 1996, 36, 476–485. [Google Scholar] [CrossRef]
- Wechsler, J.; Choi, Y.-H.; Krall, J.; Ahmad, F.; Manganiello, V.C.; Movsesian, M.A. Isoforms of Cyclic Nucleotide Phosphodiesterase PDE3A in Cardiac Myocytes. J. Biol. Chem. 2002, 277, 38072–38078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhardt, R.R.; Chin, E.; Zhou, J.; Taira, M.; Murata, T.; Manganiello, V.C.; Bondy, C.A. Distinctive Anatomical Patterns of Gene Expression for CGMP-Inhibited Cyclic Nucleotide Phosphodiesterases. J. Clin. Investig. 1995, 95, 1528–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenan, Y.; Murata, T.; Shakur, Y.; Degerman, E.; Manganiello, V.C. Functions of the N-Terminal Region of Cyclic Nucleotide Phosphodiesterase 3 (PDE 3) Isoforms. J. Biol. Chem. 2000, 275, 12331–12338. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.W.; Lagranha, C.; Chen, Y.; Sun, J.; Tong, G.; Hockman, S.C.; Ahmad, F.; Esfahani, S.G.; Bae, D.H.; Polidovitch, N.; et al. Targeted Disruption of PDE3B, but Not PDE3A, Protects Murine Heart from Ischemia/Reperfusion Injury. Proc. Natl. Acad. Sci. USA 2015, 112, E2253–E2262. [Google Scholar] [CrossRef] [Green Version]
- Oikawa, M.; Wu, M.; Lim, S.; Knight, W.E.; Miller, C.L.; Cai, Y.; Lu, Y.; Blaxall, B.C.; Takeishi, Y.; Abe, J.; et al. Cyclic Nucleotide Phosphodiesterase 3A1 Protects the Heart against Ischemia-Reperfusion Injury. J. Mol. Cell. Cardiol. 2013, 64, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Beca, S.; Ahmad, F.; Shen, W.; Liu, J.; Makary, S.; Polidovitch, N.; Sun, J.; Hockman, S.; Chung, Y.W.; Movsesian, M.; et al. Phosphodiesterase Type 3A Regulates Basal Myocardial Contractility Through Interacting With Sarcoplasmic Reticulum Calcium ATPase Type 2a Signaling Complexes in Mouse Heart. Circ. Res. 2013, 112, 289–297. [Google Scholar] [CrossRef]
- Patrucco, E.; Notte, A.; Barberis, L.; Selvetella, G.; Maffei, A.; Brancaccio, M.; Marengo, S.; Russo, G.; Azzolino, O.; Rybalkin, S.D.; et al. PI3Kgamma Modulates the Cardiac Response to Chronic Pressure Overload by Distinct Kinase-Dependent and -Independent Effects. Cell 2004, 118, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Perino, A.; Ghigo, A.; Ferrero, E.; Morello, F.; Santulli, G.; Baillie, G.S.; Damilano, F.; Dunlop, A.J.; Pawson, C.; Walser, R.; et al. Integrating Cardiac PIP3 and CAMP Signaling through a PKA Anchoring Function of P110γ. Mol. Cell. 2011, 42, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Li, H.; Shakur, Y.; Hensley, J.; Hockman, S.; Kambayashi, J.; Manganiello, V.C.; Liu, Y. Role of Phosphodiesterase Type 3A and 3B in Regulating Platelet and Cardiac Function Using Subtype-Selective Knockout Mice. Cell. Signal. 2007, 19, 1765–1771. [Google Scholar] [CrossRef]
- Baim, D.S.; McDowell, A.V.; Cherniles, J.; Monrad, E.S.; Parker, J.A.; Edelson, J.; Braunwald, E.; Grossman, W. Evaluation of a New Bipyridine Inotropic Agent--Milrinone--in Patients with Severe Congestive Heart Failure. N. Engl. J. Med. 1983, 309, 748–756. [Google Scholar] [CrossRef]
- Packer, M.; Carver, J.R.; Rodeheffer, R.J.; Ivanhoe, R.J.; DiBianco, R.; Zeldis, S.M.; Hendrix, G.H.; Bommer, W.J.; Elkayam, U.; Kukin, M.L. Effect of Oral Milrinone on Mortality in Severe Chronic Heart Failure. The PROMISE Study Research Group. N. Engl. J. Med. 1991, 325, 1468–1475. [Google Scholar] [CrossRef]
- Packer, M. Effect of Phosphodiesterase Inhibitors on Survival of Patients with Chronic Congestive Heart Failure. Am. J. Cardiol. 1989, 63, 41A–45A. [Google Scholar] [CrossRef]
- Fertig, B.A.; Baillie, G.S. PDE4-Mediated CAMP Signalling. J. Cardiovasc. Dev. Dis. 2018, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostic, M.M.; Erdogan, S.; Rena, G.; Borchert, G.; Hoch, B.; Bartel, S.; Scotland, G.; Huston, E.; Houslay, M.D.; Krause, E.G. Altered Expression of PDE1 and PDE4 Cyclic Nucleotide Phosphodiesterase Isoforms in 7-Oxo-Prostacyclin-Preconditioned Rat Heart. J. Mol. Cell. Cardiol. 1997, 29, 3135–3146. [Google Scholar] [CrossRef] [PubMed]
- Leroy, J.; Richter, W.; Mika, D.; Castro, L.R.V.; Abi-Gerges, A.; Xie, M.; Scheitrum, C.; Lefebvre, F.; Schittl, J.; Mateo, P.; et al. Phosphodiesterase 4B in the Cardiac L-Type Ca2+ Channel Complex Regulates Ca2+ Current and Protects against Ventricular Arrhythmias in Mice. J. Clin. Investig. 2011, 121, 2651–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.Y.; Greenstein, J.L.; Winslow, R.L. Interaction between Phosphodiesterases in the Regulation of the Cardiac β-Adrenergic Pathway. J. Mol. Cell. Cardiol. 2015, 88, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Xie, M.; Gao, L.; Zhang, W.; Zhu, X.; Wang, Y.; Li, W.; Wang, R.; Chen, K.; Boutjdir, M.; et al. Rolipram, a PDE4 Inhibitor, Enhances the Inotropic Effect of Rat Heart by Activating SERCA2a. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Beca, S.; Helli, P.B.; Simpson, J.A.; Zhao, D.; Farman, G.P.; Jones, P.; Tian, X.; Wilson, L.S.; Ahmad, F.; Chen, S.R.W.; et al. Phosphodiesterase 4D Regulates Baseline Sarcoplasmic Reticulum Ca2+ Release and Cardiac Contractility, Independently of L-Type Ca2+ Current. Circ. Res. 2011, 109, 1024–1030. [Google Scholar] [CrossRef] [Green Version]
- Terrenoire, C.; Houslay, M.D.; Baillie, G.S.; Kass, R.S. The Cardiac IKs Potassium Channel Macromolecular Complex Includes the Phosphodiesterase PDE4D3. J. Biol. Chem. 2009, 284, 9140–9146. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Li, M.; Mika, D.; Fu, Q.; Kim, S.; Phan, J.; Shen, A.; Vandecasteele, G.; Xiang, Y.K. Heterologous Desensitization of Cardiac β-Adrenergic Signal via Hormone-Induced ΒAR/Arrestin/PDE4 Complexes. Cardiovasc. Res. 2017, 113, 656–670. [Google Scholar] [CrossRef] [Green Version]
- Lehnart, S.E.; Wehrens, X.H.T.; Reiken, S.; Warrier, S.; Belevych, A.E.; Harvey, R.D.; Richter, W.; Jin, S.-L.C.; Conti, M.; Marks, A.R. Phosphodiesterase 4D Deficiency in the Ryanodine-Receptor Complex Promotes Heart Failure and Arrhythmias. Cell 2005, 123, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Ma, T.; Wang, X. Gene Transfer of Heat-Shock Protein 20 Protects against Ischemia/Reperfusion Injury in Rat Hearts. Acta Pharm. Sin. 2005, 26, 1193–1200. [Google Scholar] [CrossRef] [Green Version]
- Nicolaou, P.; Knöll, R.; Haghighi, K.; Fan, G.-C.; Dorn, G.W.; Hasenfub, G.; Kranias, E.G. Human Mutation in the Anti-Apoptotic Heat Shock Protein 20 Abrogates Its Cardioprotective Effects. J. Biol. Chem. 2008, 283, 33465–33471. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.-C.; Chu, G.; Mitton, B.; Song, Q.; Yuan, Q.; Kranias, E.G. Small Heat-Shock Protein Hsp20 Phosphorylation Inhibits Beta-Agonist-Induced Cardiac Apoptosis. Circ. Res. 2004, 94, 1474–1482. [Google Scholar] [CrossRef] [Green Version]
- Edwards, H.V.; Scott, J.D.; Baillie, G.S. PKA Phosphorylation of the Small Heat-Shock Protein Hsp20 Enhances Its Cardioprotective Effects. Biochem. Soc. Trans. 2012, 40, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Sin, Y.Y.; Edwards, H.V.; Li, X.; Day, J.P.; Christian, F.; Dunlop, A.J.; Adams, D.R.; Zaccolo, M.; Houslay, M.D.; Baillie, G.S. Disruption of the Cyclic AMP Phosphodiesterase-4 (PDE4)-HSP20 Complex Attenuates the β-Agonist Induced Hypertrophic Response in Cardiac Myocytes. J. Mol. Cell. Cardiol. 2011, 50, 872–883. [Google Scholar] [CrossRef]
- Martin, T.P.; Hortigon-Vinagre, M.P.; Findlay, J.E.; Elliott, C.; Currie, S.; Baillie, G.S. Targeted Disruption of the Heat Shock Protein 20-Phosphodiesterase 4D (PDE4D) Interaction Protects against Pathological Cardiac Remodelling in a Mouse Model of Hypertrophy. FEBS Open Bio. 2014, 4, 923–927. [Google Scholar] [CrossRef] [Green Version]
- Lukyanenko, Y.O.; Younes, A.; Lyashkov, A.E.; Tarasov, K.V.; Riordon, D.R.; Lee, J.; Sirenko, S.G.; Kobrinsky, E.; Ziman, B.; Tarasova, Y.S.; et al. Ca2+/Calmodulin-Activated Phosphodiesterase 1A Is Highly Expressed in Rabbit Cardiac Sinoatrial Nodal Cells and Regulates Pacemaker Function. J. Mol. Cell Cardiol. 2016, 98, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Patrucco, E.; Albergine, M.S.; Santana, L.F.; Beavo, J.A. Phosphodiesterase 8A (PDE8A) Regulates Excitation–Contraction Coupling in Ventricular Myocytes. J. Mol. Cell. Cardiol. 2010, 49, 330–333. [Google Scholar] [CrossRef] [Green Version]
- Movsesian, M.A. Beta-Adrenergic Receptor Agonists and Cyclic Nucleotide Phosphodiesterase Inhibitors: Shifting the Focus from Inotropy to Cyclic Adenosine Monophosphate. J. Am. Coll. Cardiol. 1999, 34, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Kemp, C.D.; Conte, J.V. The Pathophysiology of Heart Failure. Cardiovasc. Pathol. 2012, 21, 365–371. [Google Scholar] [CrossRef]
- Schulz, R.; Rose, J.; Martin, C.; Brodde, O.E.; Heusch, G. Development of Short-Term Myocardial Hibernation. Its Limitation by the Severity of Ischemia and Inotropic Stimulation. Circulation 1993, 88, 684–695. [Google Scholar] [CrossRef] [Green Version]
- Lubbe, W.F.; Podzuweit, T.; Opie, L.H. Potential Arrhythmogenic Role of Cyclic Adenosine Monophosphate (AMP) and Cytosolic Calcium Overload: Implications for Prophylactic Effects of Beta-Blockers in Myocardial Infarction and Proarrhythmic Effects of Phosphodiesterase Inhibitors. J. Am. Coll. Cardiol. 1992, 19, 1622–1633. [Google Scholar] [CrossRef] [Green Version]
- Freemantle, N.; Cleland, J.; Young, P.; Mason, J.; Harrison, J. Beta Blockade after Myocardial Infarction: Systematic Review and Meta Regression Analysis. BMJ 1999, 318, 1730–1737. [Google Scholar] [CrossRef] [Green Version]
- Dézsi, C.A.; Szentes, V. The Real Role of β-Blockers in Daily Cardiovascular Therapy. Am. J. Cardiovasc. Drugs 2017, 17, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Bristow, M.R. Treatment of Chronic Heart Failure with β-Adrenergic Receptor Antagonists: A Convergence of Receptor Pharmacology and Clinical Cardiology. Circ. Res. 2011, 109, 1176–1194. [Google Scholar] [CrossRef] [Green Version]
- Iwai-Kanai, E.; Hasegawa, K.; Araki, M.; Kakita, T.; Morimoto, T.; Sasayama, S. Alpha- and Beta-Adrenergic Pathways Differentially Regulate Cell Type-Specific Apoptosis in Rat Cardiac Myocytes. Circulation 1999, 100, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Communal, C.; Singh, K.; Pimentel, D.R.; Colucci, W.S. Norepinephrine Stimulates Apoptosis in Adult Rat Ventricular Myocytes by Activation of the Beta-Adrenergic Pathway. Circulation 1998, 98, 1329–1334. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ke, P.; Zhang, J.; Zhang, X.; Chen, X. Protein Kinase Inhibitor Peptide as a Tool to Specifically Inhibit Protein Kinase A. Front. Physiol. 2020, 11, 574030. [Google Scholar] [CrossRef]
- Scott, J.D.; Fischer, E.H.; Demaille, J.G.; Krebs, E.G. Identification of an Inhibitory Region of the Heat-Stable Protein Inhibitor of the CAMP-Dependent Protein Kinase. Proc. Natl. Acad. Sci. USA 1985, 82, 4379–4383. [Google Scholar] [CrossRef] [Green Version]
- Wittrup, A.; Lieberman, J. Knocking down Disease: A Progress Report on SiRNA Therapeutics. Nat. Rev. Genet. 2015, 16, 543–552. [Google Scholar] [CrossRef]
- Omar, F.; Findlay, J.E.; Carfray, G.; Allcock, R.W.; Jiang, Z.; Moore, C.; Muir, A.L.; Lannoy, M.; Fertig, B.A.; Mai, D.; et al. Small-Molecule Allosteric Activators of PDE4 Long Form Cyclic AMP Phosphodiesterases. Proc. Natl. Acad. Sci. USA 2019, 116, 13320–13329. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Vargas, M.A.X.; Kapiloff, M.S.; Dodge-Kafka, K.L. Regulation of MEF2 Transcriptional Activity by Calcineurin/MAKAP Complexes. Exp. Cell. Res. 2013, 319, 447–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.S.; Lefkowitz, R.J.; Rajagopal, S. Biased Signalling: From Simple Switches to Allosteric Microprocessors. Nat. Rev. Drug Discov. 2018, 17, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.; Du, Y.; Quoyer, J.; Panettieri, R.A.; Janz, J.M.; Bouvier, M.; Kobilka, B.K.; Benovic, J.L. Development and Characterization of Pepducins as Gs-Biased Allosteric Agonists. J. Biol. Chem. 2014, 289, 35668–35684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, R.; Schilling, J.; Song, J.; Carter, R.L.; Du, Y.; Yoo, S.M.; Traynham, C.J.; Koch, W.J.; Cheung, J.Y.; Tilley, D.G.; et al. β-Arrestin-Biased Signaling through the Β2-Adrenergic Receptor Promotes Cardiomyocyte Contraction. Proc. Natl. Acad. Sci. USA 2016, 113, E4107–E4116. [Google Scholar] [CrossRef] [Green Version]
- Grisanti, L.A.; Thomas, T.P.; Carter, R.L.; de Lucia, C.; Gao, E.; Koch, W.J.; Benovic, J.L.; Tilley, D.G. Pepducin-Mediated Cardioprotection via β-Arrestin-Biased Β2-Adrenergic Receptor-Specific Signaling. Theranostics 2018, 8, 4664–4678. [Google Scholar] [CrossRef]
- Dimmeler, S. Cardiovascular Disease Review Series. EMBO Mol. Med. 2011, 3, 697. [Google Scholar] [CrossRef]
- Ruvinov, E.; Dvir, T.; Leor, J.; Cohen, S. Myocardial Repair: From Salvage to Tissue Reconstruction. Expert. Rev. Cardiovasc. 2008, 6, 669–686. [Google Scholar] [CrossRef]
- Maheshwari, R.; Tekade, M.; Sharma, P.A.; Tekade, R.K. Nanocarriers Assisted SiRNA Gene Therapy for the Management of Cardiovascular Disorders. Curr. Pharm. Des. 2015, 21, 4427–4440. [Google Scholar] [CrossRef]
- Karam, S.; Margaria, J.P.; Bourcier, A.; Mika, D.; Varin, A.; Bedioune, I.; Lindner, M.; Bouadjel, K.; Dessillons, M.; Gaudin, F.; et al. Cardiac Overexpression of PDE4B Blunts β-Adrenergic Response and Maladaptive Remodeling in Heart Failure. Circulation 2020, 142, 161–174. [Google Scholar] [CrossRef]
- Stoschitzky’, ’Kurt Individual Beta-Blockers for Individual Patients. e-J. Esc. Counc. Cardiol. Pract. 2018, 6, 8. [CrossRef] [Green Version]



| Receptors | Cardiac Function | In Myocardial Infarction | ||
|---|---|---|---|---|
| α2-AR Alpha 2 adrenergic receptor |
| [33] |
| [34] |
| β1-AR Beta 1 adrenergic receptor |
| [35] |
| [36] [37] |
| β2-AR Beta 2 adrenergic receptor |
| [35] |
| [37] [38] [39] |
| β3-AR Beta 3 adrenergic receptor |
| [40] |
| [41] [42] |
| M2R Muscarinic receptor type 2 |
| [35] |
| [43] |
| A1AR A1A Adenosine Receptor |
| [44] |
| [44] [45] |
| A2AAR A2A Adenosine Receptor |
| [44] |
| [44] [45] |
| A2BAR A2B Adenosine Receptor |
| [44] |
| [44,45] |
| A3AR A3 Adenosine Receptor |
| [44] |
| [44,45] |
| EP3 Prostaglandin EP3 Receptor |
| [35] |
| [46] [47] |
| EP4 Prostaglandin EP4 Receptor |
| [35] |
| [46] |
| GCCR Glucagon Receptor |
| [48] |
| [49] |
| GLP1R Glucagon Like Peptide 1 Receptor |
| [13] |
| [50,51] |
| AKAP | Physiological Function | MI Alteration and Therapeutic Interest | ||
|---|---|---|---|---|
| AKAP1 D-AKAP1 S-AKAP84 AKAP121 AKAP149 |
| [140] |
| [31] [151] |
| AKAP5 AKAP79 AKAP75 AKAP150 |
| [152] [153] [154] |
| [155] [156] |
| AKAP6 mAKAP AKAP100 |
| [157] [158] [159] [160] |
| [160,161] |
| AKAP8 AKAP95 |
| [162,163] |
| [164] |
| AKAP10 D-AKAP2 |
| [165] |
| [166] |
| AKAP12 Gravin AKAP250 SSeCKS |
| [167] [168] [169] [170] |
| [171] [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombe, A.-S.; Pidoux, G. Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction. Cells 2021, 10, 922. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10040922
Colombe A-S, Pidoux G. Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction. Cells. 2021; 10(4):922. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10040922
Chicago/Turabian StyleColombe, Anne-Sophie, and Guillaume Pidoux. 2021. "Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction" Cells 10, no. 4: 922. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10040922