Fabry Cardiomyopathy: Current Practice and Future Directions
Abstract
:1. Introduction
2. Clinical Presentation of Fabry Disease
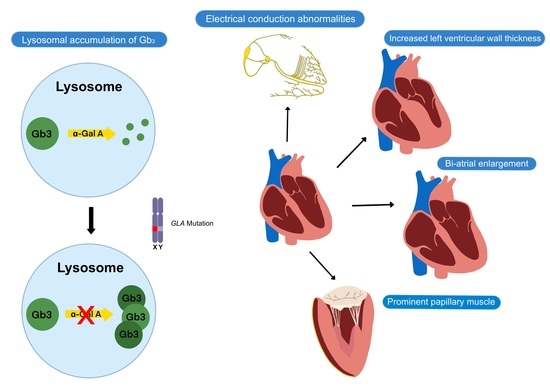
3. Fabry Cardiomyopathy
4. Screening and Diagnosis
5. Diagnosis of Fabry Cardiomyopathy
5.1. Echocardiography
5.2. Magnetic Resonance Imaging
5.3. Laboratory Tests
5.4. Cardiopulmonary Exercise Test
6. Fabry Disease Severity Scores
7. Treatments in Fabry Disease
8. Future Directions in the Management of Fabry Disease
9. Conclusions and Call to Action
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| a-Gal A | α-galactosidase A |
| ACE | Angiotensin converting enzyme |
| ARB | Angiotensin II receptor blocker |
| AI | Artificial intelligence |
| AV | Atrioventricular |
| DBS | Dried blood spot |
| ECG | Electrocardiogram |
| ERT | Enzyme replacement therapy |
| FD | Fabry disease |
| Gb3 | Globotriaosylceramide |
| HCM | Hypertrophic cardiomyopathy |
| ICD | Implantable cardioverter-defibrillator |
| LGE | Late gadolinium enhancement |
| LV | Left ventricular |
| LVEF | Left ventricular ejection fraction |
| LVOT | Left ventricular outflow tract |
| MRI | Magnetic resonance imaging |
| RV | Right ventricular |
| SRT | Substrate reduction therapy |
| VT | Ventricular tachycardia |
References
- Germain, D.P. Fabry disease. Orphanet J. Rare Dis. 2010, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwu, W.L.; Chien, Y.H.; Lee, N.C.; Chiang, S.C.; Dobrovolny, R.; Huang, A.C.; Yeh, H.Y.; Chao, M.C.; Lin, S.J.; Kitagawa, T.; et al. Newborn screening for fabry disease in taiwan reveals a high incidence of the later-onset GLA mutation c.936 + 919G > A (IVS4 + 919G > A). Hum. Mutat. 2009, 30, 1397–1405. [Google Scholar] [CrossRef] [Green Version]
- Spada, M.; Pagliardini, S.; Yasuda, M.; Tukel, T.; Thiagarajan, G.; Sakuraba, H.; Ponzone, A.; Desnick, R.J. High incidence of later-onset Fabry disease revealed by newborn screening. Am. J. Hum. Genet. 2006, 79, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Yeung, D.F.; Sirrs, S.; Tsang, M.Y.C.; Gin, K.; Luong, C.; Jue, J.; Nair, P.; Lee, P.K.; Tsang, T.S.M. Echocardiographic Assessment of Patients with Fabry Disease. J. Am. Soc. Echocardiogr. 2018, 31, 639–649.e2. [Google Scholar] [CrossRef]
- Pieroni, M.; Moon, J.C.; Arbustini, E.; Barriales-Villa, R.; Camporeale, A.; Vujkovac, A.C.; Elliott, P.M.; Hagege, A.; Kuusisto, J.; Linhart, A.; et al. Cardiac Involvement in Fabry Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 922–936. [Google Scholar] [CrossRef]
- Seydelmann, N.; Wanner, C.; Störk, S.; Ertl, G.; Weidemann, F. Fabry disease and the heart. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Chamoles, N.A.; Blanco, M.; Gaggioli, D. Fabry disease: Enzymatic diagnosis in dried blood spots on filter paper. Clin. Chim. Acta 2001, 308, 195–196. [Google Scholar] [CrossRef]
- Mehta, A.; Clarke, J.T.R.; Giugliani, R.; Elliott, P.; Linhart, A.; Beck, M.; Sunder-Plassmann, G. Natural course of Fabry disease: Changing pattern of causes of death in FOS-Fabry Outcome Survey. J. Med. Genet. 2009, 46, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Linhart, A.; Germain, D.P.; Olivotto, I.; Akhtar, M.M.; Anastasakis, A.; Hughes, D.; Namdar, M.; Pieroni, M.; Hagège, A.; Cecchi, F.; et al. An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur. J. Heart Fail. 2020, 22, 1076–1096. [Google Scholar] [CrossRef]
- Nakao, S.; Takenaka, T.; Maeda, M.; Kodama, C.; Tanaka, A.; Tahara, M.; Yoshida, A.; Kuriyama, M.; Hayashibe, H.; Sakuraba, H.; et al. An Atypical Variant of Fabry’s Disease in Men with Left Ventricular Hypertrophy. N. Engl. J. Med. 1995, 333, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.R.; Hung, S.C.; Chang, F.P.; Yu, W.C.; Sung, S.H.; Hsu, C.L.; Dzhagalov, I.; Yang, C.F.; Chu, T.H.; Lee, H.J.; et al. Later Onset Fabry Disease, Cardiac Damage Progress in Silence: Experience With a Highly Prevalent Mutation. J. Am. Coll. Cardiol. 2016, 68, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Hughes, D.A.; Sachdev, B.; Tome, M.; Ward, D.; Lee, P.; Mehta, A.B.; Elliott, P.M. Prevalence and clinical significance of cardiac arrhythmia in Anderson-Dabry disease. Am. J. Cardiol. 2005, 96, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, F.; Sanchez-Niño, M.D.; Politei, J.; Oliveira, J.P.; Wanner, C.; Warnock, D.G.; Ortiz, A. Fibrosis: A key feature of Fabry disease with potential therapeutic implications. Orphanet J. Rare Dis. 2013, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Omahony, C.; Coats, C.; Cardona, M.; Garcia, A.; Calcagnino, M.; Murphy, E.; Robin, L.; Atul, M.; Derralynn, H.; Perry, M.E. Incidence and predictors of anti-bradycardia pacing in patients with Anderson-Fabry disease. Europace 2011, 13, 1781–1788. [Google Scholar] [CrossRef]
- Namdar, M. Electrocardiographic Changes and Arrhythmia in Fabry Disease. Front. Cardiovasc. Med. 2016, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Niemann, M.; Breunig, F.; Beer, M.; Herrmann, S.; Strotmann, J.; Hu, K.; Emmert, A.; Voelker, W.; Ertl, G.; Wanner, C.; et al. The right ventricle in Fabry disease: Natural history and impact of enzyme replacement therapy. Heart 2010, 96, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, C.; Linhart, A.; Baehner, F.; Palecek, T.; Wiethoff, C.M.; Miebach, E.; Whybra, C.; Gal, A.; Bultas, J.; Beck, M. Onset and progression of the Anderson-Fabry disease related cardiomyopathy. Int. J. Cardiol. 2008, 130, 367–373. [Google Scholar] [CrossRef]
- Mehta, J.; Moller, J.H.; Desnick, R.J.; Ph, D. Electrocardiographic and vectorcardiographic abnormalities in Fabry’s disease. Am. Heart J. 1977, 93, 699–705. [Google Scholar] [CrossRef]
- Linhart, A.; Paleček, T.; Bultas, J.; Ferguson, J.J.; Hrudová, J.; Karetová, D.; Zeman, J.; Ledvinová, J.; Poupětová, H.; Elleder, M.; et al. New insights in cardiac structural changes in patients with Fabry’s disease. Am. Heart J. 2000, 139, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Barbey, F.; Qanadli, S.D.; Juli, C.; Brakch, N.; Palaek, T.; Rizzo, E.; Jeanrenaud, X.; Eckhardt, B.; Linhart, A. Aortic remodelling in Fabry disease. Eur. Heart J. 2010, 31, 347–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desnick, R.J.; Blieden, L.C.; Sharp, H.L.; Hofschire, P.J.; Moller, J.H. Cardiac valvular anomalies in Fabry disease. Clinical, morphologic, and biochemical studies. Circulation 1976, 54, 818–825. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, B.; Takenaka, T.; Teraguchi, H.; Tei, C.; Lee, P.; McKenna, W.J.; Elliott, P.M. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation 2002, 105, 1407–1411. [Google Scholar] [CrossRef] [Green Version]
- Maron, M.S.; Xin, W.; Sims, K.B.; Butler, R.; Haas, T.S.; Rowin, E.J.; Desnick, R.J.; Maron, B.J. Identification of Fabry Disease in a Tertiary Referral Cohort of Patients with Hypertrophic Cardiomyopathy. Am. J. Med. 2018, 131, 200.e1–200.e8. [Google Scholar] [CrossRef]
- Doheny, D.; Srinivasan, R.; Pagant, S.; Chen, B.; Yasuda, M.; Desnick, R.J. Fabry Disease: Prevalence of affected males and heterozygotes with pathogenic GLA mutations identified by screening renal, cardiac and stroke clinics, 1995–2017. J. Med. Genet. 2018, 55, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chong, K.W.; Hsu, J.H.; Yu, H.C.; Shih, C.C.; Huang, C.H.; Lin, S.J.; Chen, C.H.; Chiang, C.C.; Ho, H.J.; et al. High incidence of the cardiac variant of fabry disease revealed by newborn screening in the Taiwan Chinese population. Circ. Cardiovasc. Genet. 2009, 2, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Baig, S.; Edward, N.C.; Kotecha, D.; Liu, B.; Nordin, S.; Kozor, R.; Moon, J.C.; Geberhiwot, T.; Steeds, R.P. Ventricular arrhythmia and sudden cardiac death in Fabry disease: A systematic review of risk factors in clinical practice. Europace 2018, 20, f153–f161. [Google Scholar] [CrossRef]
- Echevarria, L.; Benistan, K.; Toussaint, A.; Dubourg, O.; Hagege, A.A.; Eladari, D.; Jabbour, F.; Beldjord, C.; De Mazancourt, P.; Germain, D.P. X-chromosome inactivation in female patients with Fabry disease. Clin. Genet. 2016, 89, 44–54. [Google Scholar] [CrossRef]
- Meikle, P.J.; Hopwood, J.J.; Clague, A.E.; Carey, W.F. Prevalence of lysosomal storage disorders. J. Am. Med. Assoc. 1999, 281, 249–254. [Google Scholar] [CrossRef]
- Mundigler, G.; Gaggl, M.; Heinze, G.; Graf, S.; Zehetgruber, M.; Lajic, N.; Voigtlander, T.; Mannhalter, C.; Sunder-Plassmann, R.; Paschke, E.; et al. The endocardial binary appearance (‘binary sign’) is an unreliable marker for echocardiographic detection of Fabry disease in patients with left ventricular hypertrophy. Eur. J. Echocardiogr. 2011, 12, 744–749. [Google Scholar] [CrossRef] [Green Version]
- Gruner, C.; Verocai, F.; Carasso, S.; Vannan, M.A.; Jamorski, M.; Clarke, J.T.R.; Care, M.; Iwanochko, R.M.; Rakowski, H. Systolic myocardial mechanics in patients with Anderson-Fabry disease with and without left ventricular hypertrophy and in comparison to nonobstructive hypertrophic cardiomyopathy. Echocardiography 2012, 29, 810–817. [Google Scholar] [CrossRef]
- Labombarda, F.; Saloux, E.; Milesi, G.; Bienvenu, B. Loss of base-to-apex circumferential strain gradient: A specific pattern of Fabry cardiomyopathy? Echocardiography 2017, 34, 504–510. [Google Scholar] [CrossRef]
- Hindieh, W.; Weissler-Snir, A.; Hammer, H.; Adler, A.; Rakowski, H.; Chan, R.H. Discrepant Measurements of Maximal Left Ventricular Wall Thickness Between Cardiac Magnetic Resonance Imaging and Echocardiography in Patients With Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Huang, H.; Yu, Q.; Ma, Y.; Zhang, Q.; Zhang, B. Artificial intelligence-based myocardial texture analysis in etiological differentiation of left ventricular hypertrophy. Ann. Transl. Med. 2021, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Mahara, K.; Beussink-Nelson, L.; Ikura, H.; Katsumata, Y.; Endo, J.; Gaggin, H.K.; Shah, S.J.; Itabashi, Y.; MacRae, C.A.; et al. Artificial intelligence-enabled fully automated detection of cardiac amyloidosis using electrocardiograms and echocardiograms. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Augusto, J.B.; Davies, R.H.; Bhuva, A.N.; Knott, K.D.; Seraphim, A.; Alfarih, M.; Lau, C.; Hughes, R.K.; Lopes, L.R.; Shiwani, H.; et al. Diagnosis and risk stratification in hypertrophic cardiomyopathy using machine learning wall thickness measurement: A comparison with human test-retest performance. Lancet Digit. Health 2021, 3, e20–e28. [Google Scholar] [CrossRef]
- Zhang, J.; Deo, R.C. Response by Zhang and Deo to Letter Regarding Article, “Fully Automated Echocardiogram Interpretation in Clinical Practice: Feasibility and Diagnostic Accuracy”. Circulation 2019, 139, 1648–1649. [Google Scholar] [CrossRef]
- Sado, D.M.; White, S.K.; Piechnik, S.K.; Banypersad, S.M.; Treibel, T.; Captur, G.; Fontana, M.; Maestrini, V.; Flett, A.S.; Robson, M.D.; et al. Identification and assessment of anderson-fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ. Cardiovasc. Imaging 2013, 6, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Pica, S.; Sado, D.M.; Maestrini, V.; Fontana, M.; White, S.K.; Treibel, T.; Captur, G.; Anderson, S.; Piechnik, S.K.; Robson, M.D.; et al. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 99. [Google Scholar] [CrossRef]
- Augusto, J.B.; Nordin, S.; Vijapurapu, R.; Baig, S.; Bulluck, H.; Castelletti, S.; Alfarih, M.; Knott, K.; Captur, G.; Kotecha, T.; et al. Myocardial edema, myocyte injury, and disease severity in Fabry disease. Circ. Cardiovasc. Imaging 2020, 13, 10171. [Google Scholar] [CrossRef]
- Seydelmann, N.; Liu, D.; Krämer, J.; Drechsler, C.; Hu, K.; Nordbeck, P.; Schneider, A.; Störk, S.; Bijnens, B.; Ertl, G.; et al. High-sensitivity troponin: A clinical blood biomarker for staging cardiomyopathy in fabry disease. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Yogasundaram, H.; Nikhanj, A.; Putko, B.N.; Boutin, M.; Jain-Ghai, S.; Khan, A.; Auray-Blais, C.; West, M.L.; Oudit, G.Y. Elevated inflammatory plasma biomarkers in patients with fabry disease: A critical link to heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altarescu, G.; Chicco, G.; Whybra, C.; Delgado-Sanchez, S.; Sharon, N.; Beck, M.; Elstein, D. Correlation between interleukin-6 promoter and C-reactive protein (CRP) polymorphisms and CRP levels with the Mainz Severity Score Index for Fabry disease. J. Inherit. Metab. Dis. 2008, 31, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Coats, C.J.; Parisi, V.; Ramos, M.; Janagarajan, K.; O’Mahony, C.; Dawnay, A.; Lachmann, R.H.; Murphy, E.; Mehta, A.; Hughes, D.; et al. Role of serum N-terminal pro-brain natriuretic peptide measurement in diagnosis of cardiac involvement in patients with anderson-fabry disease. Am. J. Cardiol. 2013, 111, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Lobo, T.; Morgan, J.; Bjorksten, A.; Nicholls, K.; Grigg, L.; Centra, E.; Becker, G. Cardiovascular testing in Fabry disease: Exercise capacity reduction, chronotropic incompetence and improved anaerobic threshold after enzyme replacement. Intern. Med. J. 2008, 38, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Bierer, G.; Kamangar, N.; Balte, D.; Wilcox, W.R.; Mosenifar, Z. Cardiopulmonary exercise testing in fabry disease. Respiration 2005, 72, 504–511. [Google Scholar] [CrossRef]
- Powell, A.W.; Jefferies, J.L.; Hopkin, R.J.; Mays, W.A.; Goa, Z.; Chin, C. Cardiopulmonary fitness assessment on maximal and submaximal exercise testing in patients with Fabry disease. Am. J. Med. Genet. Part A 2018, 176, 1852–1857. [Google Scholar] [CrossRef]
- Bierer, G.; Balfe, D.; Wilcox, W.R.; Mosenifar, Z. Improvement in serial cardiopulmonary exercise testing following enzyme replacement therapy in Fabry disease. J. Inherit. Metab. Dis. 2006, 29, 572–579. [Google Scholar] [CrossRef]
- Giannini, E.H.; Mehta, A.B.; Hilz, M.J.; Beck, M.; Bichet, D.G.; Brady, R.O.; West, M.; Germain, D.P.; Wanner, C.; Waldek, S.; et al. A validated disease severity scoring system for Fabry disease. Mol. Genet. Metab. 2010, 99, 283–290. [Google Scholar] [CrossRef]
- Whybra, C.; Kampmann, C.; Krummenauer, F.; Ries, M.; Mengel, E.; Miebach, E.; Baehner, F.; Kim, K.; Bajbouj, M.; Schwarting, A.; et al. The Mainz Severity Score Index: A new instrument for quantifying the Anderson-Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin. Genet. 2004, 65, 299–307. [Google Scholar] [CrossRef]
- Mignani, R.; Pieruzzi, F.; Berri, F.; Burlina, A.; Chinea, B.; Gallieni, M.; Pieroni, M.; Salviati, A.; Spada, M. FAbry STabilization indEX (FASTEX): An innovative tool for the assessment of clinical stabilization in Fabry disease. Clin. Kidney J. 2016, 9, 739–747. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Barber, D.L.; Huang, J.; Rupar, C.A.; Rip, J.W.; Auray-Blais, C.; Boutin, M.; O’Hoski, P.; Gargulak, K.; McKillop, W.M.; et al. Lentivirus-mediated gene therapy for Fabry disease. Nat. Commun. 2021, 12. [Google Scholar] [CrossRef]
- Eng, C.M.; Guffon, N.; Wilcox, W.R.; Germain, D.P.; Lee, P.; Waldek, S.; Caplan, L.; Linthorst, G.E.; Desnick, R.J. Safety and Efficacy of Recombinant Human α-Galactosidase A Replacement Therapy in Fabry’s Disease. N. Engl. J. Med. 2001, 345, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Weidemann, F.; Niemann, M.; Breunig, F.; Herrmann, S.; Beer, M.; Störk, S.; Voelker, W.; Ertl, G.; Wanner, C.; Strotmann, J. Long-term effects of enzyme replacement therapy on fabry cardiomyopathy. Evidence for a better outcome with early treatment. Circulation 2009, 119, 524–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidemann, F.; Breunig, F.; Beer, M.; Sandstede, J.; Turschner, O.; Voelker, W.; Ertl, G.; Knoll, A.; Wanner, C.; Strotmann, J.M. Improvement of cardiac function during enzyme replacement therapy in patients with fabry disease: A prospective strain rate imaging study. Circulation 2003, 108, 1299–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Veen, S.J.; Hollak, C.E.M.; Van Kuilenburg, A.B.P.; Langeveld, M. Developments in the treatment of Fabry disease. J. Inherit. Metab. Dis. 2020, 43, 908–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiffmann, R.; Kopp, J.B.; Austin, H.A.; Balow, J.E.; Brady, R.O. Enzyme Replacement Therapy in Fabry Disease: A Randomized Controlled Trial. N. Engl. J. Med. 2001, 285, 2743. [Google Scholar] [CrossRef] [PubMed]
- El Dib, R.; Gomaa, H.; Carvalho, R.P.; Camargo, S.E.; Bazan, R.; Barretti, P.; Barreto, F.C. Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Sirrs, S.M.; Bichet, D.G.; Casey, R.; Clarke, J.T.R.; Lemoine, K.; Doucette, S.; West, M.L. Outcomes of patients treated through the Canadian Fabry disease initiative. Mol. Genet. Metab. 2014, 111, 499–506. [Google Scholar] [CrossRef]
- Arends, M.; Biegstraaten, M.; Wanner, C.; Sirrs, S.; Mehta, A.; Elliott, P.M.; Oder, D.; Watkinson, O.T.; Bichet, D.G.; Khan, A.; et al. Agalsidase alfa versus agalsidase beta for the treatment of Fabry disease: An international cohort study. J. Med. Genet. 2018, 55, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Germain, D.P.; Hughes, D.A.; Nicholls, K.; Bichet, D.G.; Giugliani, R.; Wilcox, W.R.; Feliciani, C.; Shankar, S.P.; Ezgu, F.; Amartino, H.; et al. Treatment of Fabry’s Disease with the Pharmacologic Chaperone Migalastat. N. Engl. J. Med. 2016, 375, 545–555. [Google Scholar] [CrossRef]
- Germain, D.P.; Fan, J.Q. Pharmacological chaperone therapy by active-site-specific chaperones in Fabry disease: In vitro and preclinical studies. Int. J. Clin. Pharmacol. Ther. 2009, 47, S111–S117. [Google Scholar] [PubMed]
- Hughes, D.A.; Nicholls, K.; Shankar, S.P.; Sunder-Plassmann, G.; Koeller, D.; Nedd, K.; Vockley, G.; Hamazaki, T.; Lachmann, R.; Ohashi, T.; et al. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J. Med. Genet. 2017, 54, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P.; Nicholls, K.; Giugliani, R.; Bichet, D.G.; Hughes, D.A.; Barisoni, L.M.; Colvin, R.B.; Jennette, J.C.; Skuban, N.; Castelli, J.P.; et al. Efficacy of the pharmacologic chaperone migalastat in a subset of male patients with the classic phenotype of Fabry disease and migalastat-amenable variants: Data from the phase 3 randomized, multicenter, double-blind clinical trial and extension study. Genet. Med. 2019, 21, 1987–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashe, K.M.; Budman, E.; Bangari, D.S.; Siegel, C.S.; Nietupski, J.B.; Wang, B.; Desnick, R.J.; Scheule, R.K.; Leonard, J.P.; Cheng, S.H.; et al. Efficacy of enzyme and substrate reduction therapy with a novel antagonist of glucosylceramide synthase for fabry disease. Mol. Med. 2015, 21, 389–399. [Google Scholar] [CrossRef]
- Nabhan, J.F.; Wood, K.M.; Rao, V.P.; Morin, J.; Bhamidipaty, S.; Labranche, T.P.; Gooch, R.L.; Bozal, F.; Bulawa, C.E.; Guild, B.C. Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich’s ataxia. Sci. Rep. 2016, 6, 20019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabregat-Andrés, Ó.; Pina-Buded, S.; Valverde-Navarro, A.A. Feasibility and diagnostic performance of including point-of-care ultrasound (POCUS) in preparticipation screening of young competitive athletes. Cardiol. Young 2020, 30, 1970–1972. [Google Scholar] [CrossRef]
- Fox, J.C.; Lahham, S.; Maldonado, G.; Klaus, S.; Aish, B.; Sylwanowicz, L.V.; Yanuck, J.; Wilson, S.P.; Shieh, M.; Anderson, C.L.; et al. Hypertrophic Cardiomyopathy in Youth Athletes: Successful Screening with Point-of-Care Ultrasound by Medical Students: Successful. J. Ultrasound Med. 2017, 36, 1109–1115. [Google Scholar] [CrossRef]
- Moulson, N.; Jaff, Z.; Wiltshire, V.; Taylor, T.; O’Connor, H.M.; Hopman, W.M.; Johri, A.M. Feasibility and Reliability of Nonexpert POCUS for Cardiovascular Preparticipation Screening of Varsity Athletes: The SHARP Protocol. Can. J. Cardiol. 2019, 35, 35–41. [Google Scholar] [CrossRef]
- Ko, W.Y.; Siontis, K.C.; Attia, Z.I.; Carter, R.E.; Kapa, S.; Ommen, S.R.; Demuth, S.J.; Ackerman, M.J.; Gersh, B.J.; Arruda-Olson, A.M.; et al. Detection of Hypertrophic Cardiomyopathy Using a Convolutional Neural Network-Enabled Electrocardiogram. J. Am. Coll. Cardiol. 2020, 75, 722–733. [Google Scholar] [CrossRef]
- Martini, N.; Aimo, A.; Barison, A.; Della Latta, D.; Vergaro, G.; Aquaro, G.D.; Ripoli, A.; Emdin, M.; Chiappino, D. Deep learning to diagnose cardiac amyloidosis from cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2020, 22. [Google Scholar] [CrossRef]

| Structural abnormalities detected by cardiac imaging |
|
| Electrophysiologic abnormalities detected by ECG or prolonged rhythm monitoring |
|
| Patient History | ECG | Echocardiography | CMR | |
|---|---|---|---|---|
| Fabry Cardiomyopathy |
|
|
|
|
| Hypertension |
|
|
|
|
| Athlete’s Heart |
|
|
|
|
| Aortic Stenosis |
|
|
|
|
| Hypertrophic Cardiomyopathy |
|
|
|
|
| Cardiac Amyloidosis |
|
|
|
|
| Disease-Modifying Therapy | Advantages | Disadvantages |
|---|---|---|
First-generation ERT
|
|
|
Oral chaperone therapy
|
|
|
Second-generation ERT
|
|
|
Substrate reduction therapy
|
|
|
| Gene therapy |
|
|
| Structural abnormalities that can be present on cardiac imaging |
|
| Electrophysiologic abnormalities detected by ECG or rhythm monitoring |
|
| Other cardiovascular considerations in patients with Fabry disease |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yim, J.; Yau, O.; Yeung, D.F.; Tsang, T.S.M. Fabry Cardiomyopathy: Current Practice and Future Directions. Cells 2021, 10, 1532. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10061532
Yim J, Yau O, Yeung DF, Tsang TSM. Fabry Cardiomyopathy: Current Practice and Future Directions. Cells. 2021; 10(6):1532. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10061532
Chicago/Turabian StyleYim, Jeffrey, Olivia Yau, Darwin F. Yeung, and Teresa S. M. Tsang. 2021. "Fabry Cardiomyopathy: Current Practice and Future Directions" Cells 10, no. 6: 1532. https://0-doi-org.brum.beds.ac.uk/10.3390/cells10061532