1. Introduction
Rheumatoid arthritis (RA) is a chronic form of autoimmune inflammatory disease [
1]. RA affects an estimated 0.2–1% of the global population, including 0.28–0.41% of individuals in China, and ~80% of affected patients are female [
2,
3]. RA patients experience bone erosion and persistent inflammation of the joints characterized caused by immune cells and fibroblasts present within the synovial tissues [
4]. The progressive erosion of joint tissue and consequent joint bone destruction ultimately cause the incapacitation of individuals with RA [
5]. As such, further studies of the inflammatory and immune responses that occur within the synovial microenvironment in RA patients are vital in order to better guide the treatment of this debilitating disease.
The T cell co-stimulatory molecule OX40 and its cognate ligand OX40L have attracted broad research interest as therapeutic targets in T cell-mediated diseases [
6]. OX40/OX40L is a key regulator of both innate and adaptive immunity and is capable of regulating both macrophage and T cell function [
7]. In RA, the OX40/OX40L pathway plays an important role. In RA models and RA patients, OX40 is involved in the development of RA mediated by T lymphocytes [
8]. OX40L mAb administration to type II collagen (CII) immunized DBA/1 mice significantly improved disease severity [
9]. T lymphocytes in synovial fluid and synovial tissue from RA patients express OX40, and OX40L is expressed on sub-lining cells in synovial tissue [
9]. OX40-Fc fusion protein alleviates PD-1-Fc-exacerbated RA by suppressing the inflammatory response [
10]. In addition, OX40 plays a pathogenic role in the development of autoimmune arthritis as an alternative co-stimulator of CD4
+CD28
− T cells, suggesting it as a potential target for immunomodulatory therapy in RA [
11]. These data suggest that OX40/OX40L plays a key role in the development of RA and that the OX40/OX40L pathway in T cells may be a potential target for the treatment of RA.
CD4
+ T cells are key mediators of RA pathogenesis and important components of the joint microenvironment in affected patients [
12]. While many researchers have focused on the specific roles that these CD4
+ T cells play in RA, how interactions between these cells and synovial macrophages (SMs) contribute to RA progression remains poorly understood. Many different factors control the differentiation of CD4
+ T cells, including T cell receptor signaling and major histocompatibility complex (MHC)-mediated antigen presentation [
13,
14]. Tumor necrosis factor (TNF) superfamily proteins are also key regulators of CD4
+ T cell differentiation [
6], with the OX40/OX40L signaling pathway playing a central role in the activation of particular CD4
+ T cell subsets [
15], as confirmed through studies conducted using OX40L
−/− or OX40
−/− mice [
16]. OX40L expression is evident on T cells, innate lymphoid cells, and a range of antigen-presenting cell (APC) types [
17]. Efforts to clarify the importance of OX40/OX40L signaling interactions between CD4
+ T cells and SMs within the joint microenvironment thus have the potential to better clarify the molecular pathogenesis of RA.
In the context of autoimmune disease, OX40/OX40L signaling can regulate the induction of CD4
+ T cell responses, including regulatory T cells (Tregs), T follicular helper (Tfh) cells, and type 1 helper T (Th1) cells [
18,
19,
20]. This study was designed to explore the OX40/OX40L signaling that occurs between macrophages and CD4
+ T cell subsets within the joint microenvironment. Together, these analyses have the potential to offer new insight regarding the molecular etiology of RA-related joint damage.
2. Materials and Methods
2.1. Ethics Approval
All animal studies were performed in accordance with appropriate ethical guidelines and were approved by the Institutional Animal Care and Use Committee of Zhejiang Laboratory Animal Center (approval no. ZJCLA-IACUC-20040013). Human tissue samples were collected from patients with RA or osteoarthritis (OA) who provided full informed consent to participate. All studies involving patient samples were approved by the Ethics Committee of Women’s Hospital Zhejiang University School of Medicine (approval no. IRB-20200355-R).
2.2. Mice
Female DBA/1 mice 5–8 weeks old were purchased from GemPharmatech Co., Ltd. (Nanjing, China). OX40−/− mice were purchased from GemPharmatech Co., Ltd. (Nanjing, China). Tnfsf4fl/fl mice and B6/JGpt-Lyz2em1Cin(Cre)/Gpt mice were purchased from GemPharmatech Co., Ltd. (Nanjing, China). The Lyz2-Cre strain uses a promoter specific to myeloid cells (monocytes, mature macrophages, granulocytes) to drive codon-optimized Cre (iCre). This strain specifically expresses Cre protein in myeloid cells and the mice targeted can be used as Cre tool mice for the induction of LoxP recombination in myeloid cells. Lyz2-cre mice were bred with conditional knockout model mice to delete the gene fragment between two LoxP. Therefore, mating Tnfsf4fl/fl mice and B6/JGpt-Lyz2em1Cin(Cre)/Gpt mice results in mice with conditional deficiency of OX40L in monocytes/macrophages.
2.3. Human Samples
From September 2019 to August 2021, Clinical samples were obtained from 19 OA patients and 16 RA patients (patients undergoing joint replacement), including 7 and 9 with recurrent- and progressive-type disease, respectively, defined according to the clinical classification criteria of the American Rheumatism Association for knee OA [
21,
22]. Inclusion criteria for OA: (a) Patients diagnosed with TMJ-OA according to DC/TMD diagnostic criteria; (b) patients should be ≥18 years old at the time of signing the informed consent form, regardless of gender; (c) 18.5 kg/m
2 ≤ body mass index (BMI) ≤ 35 kg/m
2, and weight ≥ 50 kg for men and ≥45 kg for women; (d) no TMD-related treatment; (e) patients fully understand the purpose and requirements of the trial, voluntarily participate in the clinical trial, and sign a written informed consent. Exclusion criteria for OA: (a) Unable to walk independently, unable to participate in the study due to dysfunction; (b) knee pain caused by trauma; (c) history of knee surgery; (d) history of rheumatoid arthritis. Inclusion criteria for RA: (a) Age 18 to 65 years, regardless of gender; (b) meet the 2010 American College of Rheumatology (ACR)/European League for Rheumatology (EULAR) classification criteria with an ACR functional classification of I-III; (c) at screening, active RA is defined as at least 6/68 joints with pressure or pain on movement and at least 4/66 joints with swelling; (d) at screening, erythrocyte sedimentation rate (ESR) ≥ upper limit of normal (ULN), or C-reactive protein (CRP) > upper limit of normal (ULN); (e) body mass index [BMI = weight/height squared (kg/m
2)] within the range of 18–30; (f) fully informed about the study, participate voluntarily, and have signed a written informed consent form. Exclusion criteria for RA: (a) Other types of arthritis (such as primary arthritis, post-traumatic osteoarthritis, gouty osteoarthritis, hemophilic osteoarthritis, and tuberculous arthritis); (b) bilateral knee arthroplasty (RA patients); (c) severe cardiovascular disease (such as myocardial infarction, atrial fibrillation, angina pectoris, and cardiac failure) or cerebrovascular disease (such as cerebral infarction and cerebral hemorrhage); (d) treated with bDMARD within 6 months, prolonged use of oral anticoagulant drugs (such as aspirin, warfarin, and clopidogrel). Participants’ age, smoking, BMI (body mass index), alcohol consumption, common chronic conditions (e.g., diabetes and hypertension), and drug use did not differ significantly between the RA group and osteoarthritis patients group (OA group) (
Supplementary Table S1).
2.4. Induction of Collagen-Induced Arthritis and Animal Samples Collection
A murine collagen-induced arthritis (CIA) model was established as in prior reports [
1]. Briefly, mice received primary and secondary immunizations on days 0 and 21, respectively. For mice in the CIA model group, these immunizations consisted of 0.1 mL of complete Freund’s adjuvant (containing 2 mg/mL chicken type II collagen (Chondrex, America, Catalog # 20011) and 4 mg/mL BCG (Hangzhou Prevention Centre, Hangzhou, China)). Beginning on day 28, two researchers independently assessed the arthritic symptoms and body weight of each mouse in this study. Arthritis severity was scored as follows: 0—normal, no joint swelling; 1—mild ankle joint or wrist swelling, or obvious swelling of the fingers; 2—moderate ankle joint or wrist swelling; 3—severe redness and swelling of the whole paw; 4—severe redness and swelling affecting more than 1 joint. Scores for each paw were summed to produce an overall score for each mouse, with a maximum possible score of 16. Mice were euthanized on day 49, at which time the spleens, hind legs, and synovial tissues of these animals were harvested for analysis.
2.5. Cell Sorting
Peripheral blood and single-cell suspensions of synovial tissues from RA patients and OA patients were used for cell sorting. The kit used for the isolation of peripheral blood mononuclear cells (PBMCs) was purchased from Miltenyi (cat. # 130-115-169). Tissue homogenizer (Servicebio, cat. # KZ-II) was used to make single-cell suspensions of synovial tissue for subsequent sorting. To ensure cell activity, we took the following measures: (1) When preparing single cells, we always ensured that the cells were in a 4 °C environment; (2) when resuspending cells, 1–2% fetal bovine serum was added to PBS; (3) when loading samples, the cells were exposed to room temperature for as little time as possible; (4) before sorting, cells were washed with EDTA-containing PBS to remove calcium ions, and DNase I (1 mg/mL) was added to remove adhesions from dead cell DNA; (5) PI staining was used to remove dead cells. Positive sorting was performed by labeling cells with appropriate surface antibodies, followed by flow cytometry-based analyses. The specimen should be fresh and should not contain more than 10% dead cells and debris. Positive sorting steps were as follows. (1) Centrifuge at 300× g for 10 min and carefully remove the supernatant as before. (2) Add antibody (10 μL/107 cells) according to instructions and incubate at 4 °C for 15 min in the dark. (3) Add 10–20 times the labeling volume of buffer, wash the cells, centrifuge at 300× g for 10 min, remove the supernatant, and repeat the wash 1 time. Add 40 μL of beads per 108 cells, add 960 μL of buffer, and incubate for 15 min at 4 °C, protected from light. Wash by centrifugation at 300× g. Remove supernatant and resuspend with buffer (500 μL/108 cells). (4) Perform magnetic sorting with a positive sorting column, using a Midi head and an LS sorting column. The magnetic beads used for this study were from Miltenyi, and included the following: Human CD19 MicroBeads (cat. # 130-050-301) (B cells), Human CD141 MicroBeads (130-090-512) (DCs), Human CD14 MicroBeads (cat. # 130-050-201) (monocytes), Human F4/80 MicroBeads (cat. # 130-110-443) (monocytes).
2.6. Flow Cytometry
Flow cytometry was used to analyze sorted cells (Human CD19+ B cells, Human CD141+ DCs, Human CD14+ monocytes, Human F4/80+ monocytes) or single-cell suspension of peripheral blood mononuclear cells or single-cell suspension of synovial tissues or single-cell suspension of spleen. Tissue homogenizer (Servicebio, cat. # KZ-II) was used to make single-cell suspensions of spleen. Cells were suspended in 100 μL PBS and stained with appropriate cell surface antibodies (0.5–1.5 μL) for 30 min at 4 °C in the dark. Cells were then rinsed two times using PBS and resuspended in 100 μL of PBS for analysis with a flow cytometer. Antibodies used for this analysis included the following: Hu CD11b APC M1/70 (BD Pharmingen, Cat. # 553312), Hu CD192 BV480 LS132.1D9 (BD Pharmingen, Cat. # 747852), Hu CD11c PE B-ly6 (BD Pharmingen, Cat. # 555392), Hu CD19 FITC HIB19 (BD Pharmingen, Cat. # 555412), Hu CD14 IHC Pure M5E2 (BD Pharmingen, Cat. # 550376), Hu CD183 BV480 (BD Pharmingen, Cat. # 746283), T-BET PE 4B10 (BD Pharmingen, Cat. # 561265) (Suitable for humans and mouse), Hu CD185 Alexa (BD Pharmingen, Cat. # 558113), Hu BATF PE (BD Pharmingen, Cat. # 27120S), Hu CD4 FITC (BD Pharmingen, Cat. # 550628), Hu CD25 APC (BD Pharmingen, Cat. # 560987), Hu Foxp3 PE (BD Pharmingen, Cat. # 560046), Ms CD183 BV750 (BD Pharmingen, Cat. # 747298), Ms CD185 APC (BD Pharmingen, Cat. # 560615), BATF PE (BD Pharmingen, Cat. # 564503), Ms CD25 APC (BD Pharmingen, Cat. # 557192), Ms Foxp3 PE (BD Pharmingen, Cat. # 560408), Ms CD4 FITC (BD Pharmingen, Cat. # 553046).
2.7. RT-qPCR
The sorted cells were used for the RT-qPCR analysis. Then, total RNA was extracted from the cells for RT-qPCR experiments. In this study, Trizol was used to extract total RNA. In single-cell suspensions, Trizol preserves RNA integrity while destroying cells and lysing cellular components. After chloroform was added and centrifuged, the lysate was stratified into aqueous and organic phases, with RNA present in the aqueous phase. Total RNA was quantified by a Thermo Scientific NanodROP 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The extracted RNA was then reverse-transcribed using the TAKARA (Japan) reverse transcription kit to synthesize cDNA. The reactions were prepared using SYBRGreen qPCR Master Mix® (TAKARA, Shiga, Japan) in a Pikoreal 96 Realtime PCR System (Thermo Scientific, USA) according to the instructions of the kit to detect the level of mRNA. The first-strand cDNA was synthesized using the Prime Script RT® kit (Takara, Shiga, Japan) according to the instructions. Reverse transcription conditions: 15 min at 37 °C, 5 s at 85 °C, 10 min at 37 °C. RT-qPCR was performed using the one-step method Thermo Step One® at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 40 cycles at 60 °C, and approximately 1 min at 60 °C. The Bio-Rad PCR platform (T100PCR) was used. The number of technical replicates was 3. CT values of target and internal reference genes were obtained after real-time amplification. GAPDH is used as an internal standard. Primer information of Tnfsf4 (5’→3’): forward primer GGTCAGGTCTGTCAACTCCTT, reverse primer CATCCAGGGAGGTATTGTCAGT. Relative expression was compared using the ΔΔCT method with appropriate normalization as follows: A = CT(target gene, experimental sample) − CT(internal standard gene, experimental sample), B = CT(target gene, control sample) − CT(internal standard gene, control sample), K = A − B, relative target gene expression = 2 − K.
2.8. Histology
Hind leg and spleen tissue samples harvested from mice were fixed for 24 h in 4% paraformaldehyde. Spleen samples were then immediately paraffinized. Hind limb samples were decalcified for 1 month prior to paraffinization and isolation of the knee and ankle joints. The tissue was placed in a perforated PE tube and then placed in a decalcifying bucket, poured full of EDTA decalcifying solution, sealed, and placed in a constant temperature shaker with a decalcifying solution change cycle of 2–3 days. Paraffinized knee and ankle sections were utilized for H&E staining and safranin O-fast green staining, while spleen sections were utilized for H&E staining. Two researchers independently scored the staining results for these sections. HE staining steps were as follows. (1) Dewaxing of paraffin sections to water: sequentially put the sections into xylene Ⅰ for 20 min, xylene Ⅱ for 20 min, anhydrous ethanol Ⅰ for 5 min, anhydrous ethanol Ⅱ for 5 min, 75% ethanol for 5 min, tap water washing. (2) Hematoxylin staining: stain sections with hematoxylin solution for 3–5 min, rinse with tap water. Then, treat the section with hematoxylin differentiation solution, rinse with tap water. Treat the section with hematoxylin Scott tap bluing, rinse with tap water. (3) Eosin staining: 85% ethanol for 5 min; 95% ethanol for 5 min. Finally stain sections with eosin dye for 5 min. (4) Dehydration and sealing: dehydrate as follows: 100% ethanol I for 5 min, 100% ethanol II for 5 min, 100% ethanol III for 5 min, xylene I for 5 min, xylene II for 5 min, and finally seal with neutral gum. (5) Microscopic examination, image acquisition and analysis. Safranin O-fast green staining steps were as follows. (1) Paraffin sections dewaxed to water: sequentially put the sections into environmentally friendly dewaxing transparent solution Ⅰ for 20 min, environmentally friendly dewaxing transparent solution Ⅱ for 20 min, anhydrous ethanol Ⅰ for 5 min, anhydrous ethanol Ⅱ for 5 min, 75% ethanol for 5 min, and wash with tap water. (2) Fast green staining: section into bone tissue solid green staining solution for 1–5 min, wash with water to remove excess staining solution until the cartilage is colorless, soak in 1% hydrochloric acid ethanol for 10 s, wash slightly with tap water. (3) Saffron staining: the slides were stained in saffron dye solution for 1–5 s, and then put into four cylinders of absolute ethanol, for rapid dehydration for 5 s, 2 s, and 10 s, and kept in the fourth cylinder. (4) Transparent sealing: clean xylene transparent 5 min, neutral resin sealing. (5) Microscopic examination, image acquisition and analysis.
H&E staining scoring criteria for knee and ankle samples were based on the degree of synovial cell hyperplasia (0, no hyperplasia; 1, mild hyperplasia; 2, moderate hyperplasia; 3, severe hyperplasia), the degree of vascular hyperplasia (0, no vascular hyperplasia; 1, mild vascular hyperplasia; 2 moderate vascular hyperplasia; 3 severe vascular hyperplasia), the degree of fibrous tissue hyperplasia (0, no fibrous tissue hyperplasia; 1 mild fibrous tissue hyperplasia; 2, moderate fibrous tissue hyperplasia; 3, severe fibrous tissue hyperplasia), and the degree of lymphocytic infiltration (0, no infiltration; 1 mild infiltration; 2, moderate infiltration; 3 severe infiltration). Safranin O-fast green staining of ankle and knee sections was used to visualize bone tissue, articular cartilage, and subchondral bone structures, with adult bone and cartilage stained green and red, respectively. H&E staining of spleen samples was used to assess the numbers and sizes of germinal centers in the spleen. Scoring was performed by two independent researchers (skilled in scoring joint HE staining), and the results were taken as the mean value.
2.9. Statistical Analysis
Data are means ± standard deviation (SD) and were compared using Student’s t test (two groups) or one-way ANOVA (three or more groups) after meeting the requisite assumptions for these statistical tests. Numbers of mice in individual experiments are indicated in the figure legends, and all mouse group assignments were random. A blinded approach was used when scoring clinical and histological samples. p < 0.05 was the significance threshold, and all analyses were performed using GraphPad Prism (v 8.01) for Windows.
4. Discussion
Synovial macrophages are essential mediators of the inflammatory activity and bone damage observed in the joints of RA patients [
27]. These SM populations are highly heterogeneous such that researchers have defined particular SM subtypes within the synovium [
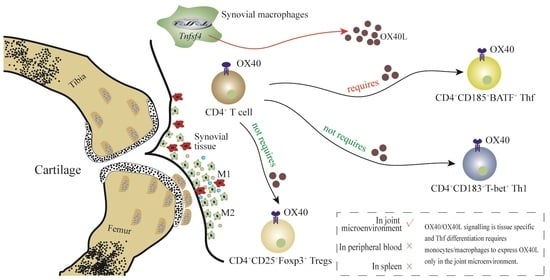
28]. The present results suggest that the expression of OX40L by SMs is vital to the effective development of Tfh within the joint microenvironment in the context of RA.
The initial analyses in this study were centered around synovial tissue samples from individuals with RA and OA based on the rationale that all medically focused research should be of clinical origin and clinically relevant where possible. OA patients served as controls in this study owing to the challenges associated with collecting synovial tissue samples from individuals without any form of joint disease. OX40L expression on the three most abundant APC populations (DCs, B cells, and monocytes/macrophages) was then analyzed in both the synovium and peripheral blood of these patients. In line with prior work, all three of these cell populations were found to exhibit increased OX40L expression in both the peripheral blood and synovium relative to OA patients. Strikingly, only OX40L expression by SMs was positively correlated with RA patient disease activity scores. These results suggest the possibility that OX40L may play a more localized role in the pathogenesis of RA, rather than shaping this disease at the systemic level. The OX40L-mediated regulation of CD4+ T cell activation may thus be a key pathway through which OX40/OX40L signaling can control RA development.
To gain further insight into the observed correlation between OX40L expression by SMs and DAS28 scores, the three CD4
+ T cell subsets most closely related to OX40/OX40L signaling (Tfh, Th1, and Tregs) were examined, given that they can control RA-related inflammatory activity and immune responses [
6,
8,
19,
29]. These analyses revealed a positive correlation between the frequency of CD185
+BATF
+ Tfh as a fraction of total CD4
+ T cells and SM OX40L expression, whereas the same correlative relationship was not detected in peripheral blood samples. This suggested the ability of OX40L expressed by SMs to control inflammation and immune response activation within the joint microenvironment through the regulation of Tfh differentiation in a tissue-specific manner. This model was further supported by the use of OX40
−/− mice, which revealed that while the impact of losing OX40 expression was systemic in a CIA model system, the corresponding impact on Tfh differentiation was tissue-specific. Specifically, the differentiation of Tfh was found to be OX40/OX40L signaling-dependent. When this signaling was no longer available, compensatory mechanisms were sufficient to sustain Tfh differentiation in the peripheral blood but not within the joint microenvironment.
To further explore the importance of OX40L expression by SMs in the context of Tfh differentiation, macrophage-specific deletion of OX40L in mice (Tnfsf4fl/fl/Lyz2-Cre mice) and control mice (Tnfsf4fl/fl mice) was used in the study. Tnfsf4 is the gene encoding the OX40L protein, and Lyz2 is predominantly expressed in macrophages. Lyz2-Cre mice were mated with Tnfsf4fl/fl mice to obtain Tnfsf4fl/fl/Lyz2-Cre mice. Our data revealed that differentiation of Tfh requires SMs to express OX40L in the articular microenvironment. Through gene editing studies in mice, our data reveal that the differentiation of Tfh requires SMs to express OX40L in the joint microenvironment. However, this phenomenon was not observed in the peripheral blood and spleen of Tnfsf4fl/fl/Lyz2-Cre mice, which laterally corroborates that the differentiation of Tfh is tissue-specific with respect to the requirement for OX40L.
Tfh are essential mediators of B cell activation and differentiation, promoting germinal center formation and immunoglobulin class switching such that they are closely related to the development of humoral immune responses [
30,
31]. Tfh differentiation occurs through pathways distinct from those employed by other related CD4
+ T cell populations such as Th1, Th17, and Tregs [
32,
33]. The process of Tfh differentiation entails initiation, maintenance, and polarization phases that rely on the coordinated activity of many surface molecules, cytokines, and transcriptional regulators [
26]. During the initiation phase, Tfh must interact with APCs. In line with these prior reports, the present data emphasize the fact that Tfh differentiation is distinct from that of Th1 and Tregs.
There are certain limitations to these analyses. For one, only specific APC subsets and CD4+ T cell subpopulations were analyzed, potentially resulting in phenotypes associated with other similar cell types having been overlooked. As such, further research will be essential to fully clarify the tissue-specific roles of OX40/OX40L signaling.