Primary Cilia and Their Role in Acquired Heart Disease
Abstract
:1. Introduction
2. Primary Cilia
2.1. Cilia Structure and Components
2.2. Ciliopathies
2.3. Primary Cilia Locations
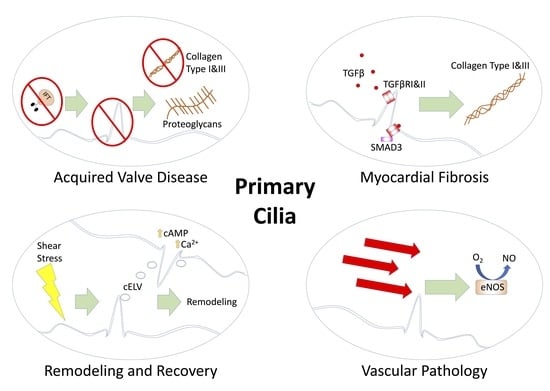
3. Primary Cilia in Acquired Heart Disease
3.1. Acquired Valvular Heart Disease
3.2. Fibrosis
3.3. Vascular Pathology and Cilia
3.4. Ventricular Remodeling and Recovery
3.5. Congenital Heart Disease and Late-Onset Heart Failure
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wu, M.H.; Lu, C.W.; Chen, H.C.; Kao, F.Y.; Huang, S.K. Adult congenital heart disease in a nationwide population 2000–2014: Epidemiological trends, arrhythmia, and standardized mortality ratio. J. Am. Heart Assoc. 2018, 7, e007907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandalenakis, Z.; Giang, K.W.; Eriksson, P.; Liden, H.; Synnergren, M.; Wåhlander, H.; Fedchenko, M.; Rosengren, A.; Dellborg, M. Survival in children with congenital heart disease: Have we reached a peak at 97%? J. Am. Heart Assoc. 2020, 9, e017704. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, S.M.; Devine, O.J.; Kucik, J.E.; Oster, M.E.; Riehle-Colarusso, T.; Nembhard, W.N.; Xu, P.; Correa, A.; Jenkins, K.; Marelli, A.J. Congenital heart defects in the United States. Circulation 2016, 134, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, T.P., Jr.; Bernard, Y.D.; Mellen, B.G.; Celermajer, D.; Baumgartner, H.; Cetta, F.; Connolly, H.M.; Davidson, W.R.; Dellborg, M.; Foster, E.; et al. Long-term outcome in congenitally corrected transposition of the great arteries: A multi-institutional study. J. Am. Coll. Cardiol. 2000, 36, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Hinton, R.B.; Ware, S.M. Heart failure in pediatric patients with congenital heart disease. Circ. Res. 2017, 120, 978–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norozi, K.; Wessel, A.; Alpers, V.; Arnhold, J.O.; Geyer, S.; Zoege, M.; Buchhorn, R. Incidence and risk distribution of heart failure in adolescents and adults with congenital heart disease after cardiac surgery. Am. J. Cardiol. 2006, 97, 1238–1243. [Google Scholar] [CrossRef]
- Oliver, J.M.; Gallego, P.; Gonzalez, A.E.; Avila, P.; Alonso, A.; Garcia-Hamilton, D.; Peinado, R.; Dos-Subirà, L.; Pijuan-Domenech, A.; Rueda, J.; et al. Predicting sudden cardiac death in adults with congenital heart disease. Heart 2021, 107, 67–75. [Google Scholar] [CrossRef]
- Budts, W.; Ravekes, W.J.; Danford, D.A.; Kutty, S. Diastolic heart failure in patients with the fontan circulation: A Review. JAMA Cardiol. 2020, 5, 590. [Google Scholar] [CrossRef]
- Inai, K. Biomarkers for heart failure and prognostic prediction in patients with Fontan circulation. Pediatr. Int. 2021. [Google Scholar] [CrossRef]
- Myklebust, R.; Engedal, H.; Saetersdal, T.S.; Ulstein, M. Primary 9+0 cilia in the embryonic and the adult human heart. Anat. Embryol. 1977, 151, 127–139. [Google Scholar] [CrossRef]
- Satir, P.; Pedersen, L.B.; Christensen, S.T. The primary cilium at a glance. J. Cell Sci. 2010, 123, 499–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, A.M.; Leaper, M.J.; Bayliss, R. The primary cilium: Guardian of organ development and homeostasis. Organogenesis 2013, 10, 62–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yang, S.; Deepak, V.; Chinipardaz, Z.; Yang, S. Identification of cilia in different mouse tissues. Cells 2021, 10, 1623. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Klena, N.T.; Gabriel, G.C.; Liu, X.; Kim, A.J.; Lemke, K.; Chen, Y.; Chatterjee, B.; Devine, W.; Damerla, R.R.; et al. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature 2015, 521, 520–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, G.C.; Young, C.B.; Lo, C.W. Role of cilia in the pathogenesis of congenital heart disease. Semin. Cell Dev. Biol. 2020, 110, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Kozminski, K.G.; Johnson, K.A.; Forscher, P.; Rosenbaum, J.L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA 1993, 90, 5519–5523. [Google Scholar] [CrossRef] [Green Version]
- Toriyama, M.; Lee, C.; Taylor, S.P.; Duran, I.; Cohn, D.H.; Bruel, A.L.; Tabler, J.M.; Drew, K.; Kelly, M.R.; Kim, S.; et al. The ciliopathy-associated CPLANE proteins direct basal body recruitment of intraflagellar transport machinery. Nat. Genet. 2016, 48, 648–656. [Google Scholar] [CrossRef] [Green Version]
- Luu, V.Z.; Luu, A.Z.; Chowdhury, B.; Elbardisy, O.; Pan, Y.; Al-Omran, M.; Quan, A.; Teoh, H.; Hess, D.A.; Verma, S. Disruption of endothelial cell intraflagellar transport protein 88 exacerbates doxorubicin-induced cardiotoxicity. Life Sci. 2020, 260, 118216. [Google Scholar] [CrossRef]
- Willaredt, M.A.; Gorgas, K.; Gardner, H.A.R.; Tucker, K.L. Multiple essential roles for primary cilia in heart development. Cilia 2012, 1, 23. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.; Moore, R.; Wang, C.; Norris, R.A. Tugging at the heart strings: The septin cytoskeleton in heart development and disease. J. Cardiovasc. Dev. Dis. 2020, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Douguet, D.; Patel, A.; Honoré, E. Structure and function of polycystins: Insights into polycystic kidney disease. Nat. Rev. Nephrol. 2019, 15, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Sakai, I.; Hasegawa, A.; Niiya, H.; Azuma, T.; Matsuo, Y.; Fujii, N.; Tanimoto, M.; Fujita, S. FLJ10849, a septin family gene, fuses MLL in a novel leukemia cell line CNLBC1 derived from chronic neutrophilic leukemia in transformation with t(4;11)(q21;q23). Leukemia 2004, 18, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Angelis, D.; Spiliotis, E.T. Septin mutations in human cancers. Front. Cell Dev. Biol. 2016, 4, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavini, I.A.; Leonardo, D.A.; Rosa, H.V.D.; Castro, D.K.S.V.; Pereira, H.D.; Valadares, N.F.; Araujo, A.P.U.; Garratt, R.C. The structural biology of septins and their filaments: An update. Front. Cell Dev. Biol. 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Kinoshita, M.; Akiyama, H.; Tomimoto, H.; Akiguchi, I.; Kumar, S.; Noda, M.; Kimura, J. Identification of septins in neurofibrillary tangles in Alzheimer’s disease. Am. J. Pathol. 1998, 153, 1551–1560. [Google Scholar] [CrossRef]
- Lovera, M.; Lüders, J. The ciliary impact of nonciliary gene mutations. Trends Cell Biol. 2021, 31, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Wheway, G.; Schmidts, M.; Mans, D.A.; Szymanska, K.; Nguyen, T.-M.T.; Racher, H.; Phelps, I.G.; Toedt, G.; Kennedy, J.; Wunderlich, K.A.; et al. An siRNA-based functional genomics screen for the identification of regulators of ciliogenesis and ciliopathy genes. Nat. Cell. Biol. 2015, 17, 1074–1087. [Google Scholar] [CrossRef]
- Van Dam, T.J.; Wheway, G.; Slaats, G.G.; Huynen, M.A.; Giles, R.H. The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium. Cilia 2013, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Van Dam, T.J.P.; Kennedy, J.; van der Lee, R.; de Vrieze, E.; Wunderlich, K.A.; Rix, S.; Dougherty, G.W.; Lambacher, N.J.; Li, C.; Jensen, V.L.; et al. CiliaCarta: An integrated and validated compendium of ciliary genes. PLoS ONE 2019, 14, e0216705. [Google Scholar] [CrossRef] [Green Version]
- Delvallée, C.; Nicaise, S.; Antin, M.; Leuvrey, A.-S.; Nourisson, E.; Leitch, C.C.; Kellaris, G.; Stoetzel, C.; Geoffroy, V.; Scheidecker, S.; et al. A BBS1 SVA F retrotransposon insertion is a frequent cause of Bardet-Biedl syndrome. Clin. Genet. 2020, 99, 318–324. [Google Scholar] [CrossRef]
- Malicki, J.J.; Johnson, C.A. The cilium: Cellular antenna and central processing unit. Trends Cell Biol. 2016, 27, 126–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pusapati, G.V.; Kong, J.; Patel, B.B.; Krishnan, A.; Sagner, A.; Kinnebrew, M.; Briscoe, J.; Aravind, L.; Rohatgi, R. CRISPR screens uncover genes that regulate target cell sensitivity to the morphogen sonic hedgehog. Dev. Cell 2018, 44, 113–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breslow, D.K.; Hoogendoorn, S.; Kopp, A.R.; Morgens, D.W.; Vu, B.K.; Kennedy, M.C.; Han, K.; Li, A.; Hess, G.T.; Bassik, M.C.; et al. A CRISPR-based screen for Hedgehog signaling provides insights into ciliary function and ciliopathies. Nat. Genet. 2018, 50, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.J.; Møller, L.B. Crosstalk of hedgehog and mTORC1 pathways. Cells 2020, 9, 2316. [Google Scholar] [CrossRef] [PubMed]
- Bangs, F.; Anderson, K.V. Primary cilia and mammalian hedgehog signaling. Cold Spring Harb. Perspect. Biol. 2017, 9, a028175. [Google Scholar] [CrossRef] [PubMed]
- Morleo, M.; Franco, B. The autophagy-cilia axis: An intricate relationship. Cells 2019, 8, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, S.C.; Anderson, K. The primary cilium: A signalling centre during vertebrate development. Nat. Rev. Genet. 2010, 11, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-E.; Kang, G.M.; Min, S.H.; Jo, D.S.; Jung, Y.-K.; Kim, K.; Kim, M.-S.; Cho, D.-H. Primary cilia mediate mitochondrial stress responses to promote dopamine neuron survival in a Parkinson’s disease model. Cell Death Dis. 2019, 10, 952. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.C.; Sul, H.J.; Hong, H.J.; Kim, K.-H.; Kero, J.; Shong, M. Loss of primary cilia promotes mitochondria-dependent apoptosis in thyroid cancer. Sci. Rep. 2021, 11, 4181. [Google Scholar] [CrossRef]
- Atkinson, K.F.; Sherpa, R.T.; Nauli, S.M. The Role of the primary cilium in sensing extracellular pH. Cells 2019, 8, 704. [Google Scholar] [CrossRef] [Green Version]
- Collins, I.; Wann, A. Regulation of the extracellular matrix by ciliary machinery. Cells 2020, 9, 278. [Google Scholar] [CrossRef] [Green Version]
- Waters, A.M.; Beales, P.L. Ciliopathies: An expanding disease spectrum. Pediatr. Nephrol. 2011, 26, 1039–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, K.; Beales, P.L. Making sense of cilia in disease: The human ciliopathies. Am. J. Med. Genet. Part C Semin. Med. Genet. 2009, 151C, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.D.; Maffei, P.; Collin, G.B.; Naggert, J.K. Alstrom syndrome: Genetics and clinical overview. Curr. Genom. 2011, 12, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Kamal, R.; Dahiya, P.; Kaur, S.; Bhardwaj, R.; Chaudhary, K. Ellis-van Creveld syndrome: A rare clinical entity. J. Oral Maxillofac. Pathol. JOMFP 2013, 17, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Keppler-Noreuil, K.M.; Adam, M.P.; Welch, J.; Muilenburg, A.; Willing, M.C. Clinical insights gained from eight new cases and review of reported cases with Jeune syndrome (asphyxiating thoracic dystrophy). Am. J. Med. Genet. Part A 2011, 155, 1021–1032. [Google Scholar] [CrossRef]
- Devi, A.R.R.; Naushad, S.M.; Lingappa, L. Clinical and molecular diagnosis of joubert syndrome and related disorders. Pediatr. Neurol. 2020, 106, 43–49. [Google Scholar] [CrossRef]
- Tsang, S.H.; Sharma, T. Leber congenital amaurosis. Adv. Exp. Med. Biol. 2018, 1085, 131–137. [Google Scholar] [CrossRef]
- Schaefer, E.; Durand, M.; Stoetzel, C.; Doray, B.; Viville, B.; Hellé, S.; Danse, J.-M.; Hamel, C.; Bitoun, P.; Goldenberg, A.; et al. Molecular diagnosis reveals genetic heterogeneity for the overlapping MKKS and BBS phenotypes. Eur. J. Med. Genet. 2011, 54, 157–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartill, V.; Szymanska, K.; Sharif, S.M.; Wheway, G.; Johnson, C.A. Meckel-Gruber syndrome: An update on diagnosis, clinical management, and research advances. Front. Pediatr. 2017, 5, 244. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.; Tao, Y. Nephronophthisis: A review of genotype–phenotype correlation. Nephrology 2018, 23, 904–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, S.H.; Aycinena, A.R.P.; Sharma, T. Ciliopathy: Senior-Løken syndrome. Adv. Exp. Med. Biol. 2018, 1085, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Walczak-Sztulpa, J.; Eggenschwiler, J.; Osborn, D.; Brown, D.A.; Emma, F.; Klingenberg, C.; Hennekam, R.C.; Torre, G.; Garshasbi, M.; Tzschach, A.; et al. Cranioectodermal dysplasia, sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am. J. Hum. Genet. 2010, 86, 949–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naki, M.M.; Gür, D.; Zemheri, E.; Tekcan, C.; Kanadikirik, F.; Has, R.; Kanadıkırık, F. Short rib-polydactyly syndrome. Arch. Gynecol. Obstet. 2004, 272, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Villalobos, E.; Criollo, A.; Schiattarella, G.; Altamirano, F.; French, K.M.; May, H.; Jiang, N.; Nguyen, N.U.N.; Romero, D.; Roa, J.C.; et al. Fibroblast primary cilia are required for cardiac fibrosis. Circulation 2019, 139, 2342–2357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chalothorn, D.; Faber, J.E. Collateral vessels have unique endothelial and smooth muscle cell phenotypes. Int. J. Mol. Sci. 2019, 20, 3608. [Google Scholar] [CrossRef] [Green Version]
- Toomer, K.A.; Yu, M.; Fulmer, D.; Guo, L.; Moore, K.S.; Moore, R.; Drayton, K.D.; Glover, J.; Peterson, N.; Ramos-Ortiz, S.; et al. Primary cilia defects causing mitral valve prolapse. Sci. Transl. Med. 2019, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; McGlashan, S.R.; Ward, M.-L. Evidence of primary cilia in the developing rat heart. Cilia 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.; Carson, J.; Lo, C. Genetics of congenital heart disease. Biomolecules 2019, 9, 879. [Google Scholar] [CrossRef] [Green Version]
- Fulmer, D.; Toomer, K.; Guo, L.; Moore, K.; Glover, J.; Moore, R.; Stairley, R.; Lobo, G.; Zuo, X.; Dang, Y.; et al. Defects in the Exocyst-Cilia machinery cause bicuspid aortic valve disease and aortic stenosis. Circulation 2019, 140, 1331–1341. [Google Scholar] [CrossRef]
- Delling, F.N.; Vasan, R.S. Epidemiology and pathophysiology of mitral valve prolapse: New insights into disease progression, genetics, and molecular basis. Circulation 2014, 129, 2158–2170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durst, R.; Sauls, K.; Peal, D.S.; de Vlaming, A.; Toomer, K.; Leyne, M.; Salani, M.; Talkowski, M.; Brand, H.; Perrocheau, M.; et al. Mutations in DCHS1 cause mitral valve prolapse. Nature 2015, 525, 109–113. [Google Scholar] [CrossRef]
- Toomer, K.A.; Fulmer, D.; Guo, L.; Drohan, A.; Peterson, N.; Swanson, P.; Brooks, B.; Mukherjee, R.; Body, S.; Lipschutz, J.H.; et al. A role for primary cilia in aortic valve development and disease. Dev. Dyn. 2017, 246, 625–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leask, A. Getting to the heart of the matter: New insights into cardiac fibrosis. Circ. Res. 2015, 116, 1269–1276. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.A.; Jerosch-Herold, M.; Hajjar, R.J. Mitral Valve Prolapse: A Disease of Valve and Ventricle. J. Am. Coll. Cardiol. 2018, 72, 835–837. [Google Scholar] [CrossRef] [PubMed]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Dobaczewski, M.; de Haan, J.J.; Frangogiannis, N.G. The Extracellular matrix modulates fibroblast phenotype and function in the infarcted myocardium. J. Cardiovasc. Transl. Res. 2012, 5, 837–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Adam, M.; Matkar, P.N.; Bugyei-Twum, A.; Desjardins, J.-F.; Chen, H.H.; Nguyen, H.; Bazinet, H.; Michels, D.; Liu, Z.; et al. Endothelial-specific Loss of IFT88 promotes endothelial-to-mesenchymal transition and exacerbates bleomycin-induced pulmonary fibrosis. Sci. Rep. 2020, 10, 4466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egorova, A.D.; Khedoe, P.P.; Goumans, M.-J.T.; Yoder, B.K.; Nauli, S.M.; Dijke, P.T.; Poelmann, R.E.; Hierck, B.P. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ. Res. 2011, 108, 1093–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spasic, M.; Jacobs, C.R. Primary cilia: Cell and molecular mechanosensors directing whole tissue function. Semin. Cell Dev. Biol. 2017, 71, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Luu, V.Z.; Chowdhury, B.; Al-Omran, M.; Hess, D.A.; Verma, S. Role of endothelial primary cilia as fluid mechanosensors on vascular health. Atherosclerosis 2018, 275, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Nauli, S.M.; Kawanabe, Y.; Kaminski, J.J.; Pearce, W.J.; Ingber, D.E.; Zhou, J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 2008, 117, 1161–1171. [Google Scholar] [CrossRef] [Green Version]
- Pala, R.; Jamal, M.; Alshammari, Q.; Nauli, S.M. The roles of primary cilia in cardiovascular diseases. Cells 2018, 7, 233. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Tempel, D.; Van Haperen, R.; Van Der Baan, A.; Grosveld, F.; Daemen, M.; Krams, R.; De Crom, R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 2006, 113, 2744–2753. [Google Scholar] [CrossRef] [Green Version]
- Van der Heiden, K.; Hierck, B.; Krams, R.; de Crom, R.; Cheng, C.; Baiker, M.; Pourquie, M.J.; Alkemade, F.E.; DeRuiter, M.C.; Gittenberger-de Groot, A.C.; et al. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis 2008, 196, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Espinha, L.C.; Hoey, D.; Fernandes, P.R.; Rodrigues, H.; Jacobs, C.R. Oscillatory fluid flow influences primary cilia and microtubule mechanics. Cytoskeleton 2014, 71, 435–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinsmore, C.; Reiter, J.F. Endothelial primary cilia inhibit atherosclerosis. EMBO Rep. 2016, 17, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Patch, C.; Charlton, J.; Roderick, P.J.; Gulliford, M.C. Use of antihypertensive medications and mortality of patients with autosomal dominant polycystic kidney disease: A population-based study. Am. J. Kidney Dis. 2011, 57, 856–862. [Google Scholar] [CrossRef]
- Abdul-Majeed, S.; Nauli, S.M. Dopamine receptor type 5 in the primary cilia has dual chemo- and mechano-sensory roles. Hypertension 2011, 58, 325–331. [Google Scholar] [CrossRef]
- Zeng, C.; Jose, P.A. Dopamine receptors. Hypertension 2011, 57, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, N.; Olson, E.N. Cardiac hypertrophy: The good, the bad, and the ugly. Annu. Rev. Physiol. 2003, 65, 45–79. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Manabe, I.; Uchino, Y.; Eguchi, K.; Matsumoto, S.; Nishimura, S.; Shindo, T.; Sano, M.; Otsu, K.; Snider, P.; et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J. Clin. Investig. 2010, 120, 254–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman, M.J.; Ganau, A.; Saba, P.S.; Pini, R.; Pickering, T.G.; Devereux, R.B. Impact of arterial stiffening on left ventricular structure. Hypertension 2000, 36, 489–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volz, A.-K.; Frei, A.; Kretschmer, V.; de Jesus Domingues, A.M.; Ketting, R.F.; Ueffing, M.; Boldt, K.; Krämer-Albers, E.-M.; May-Simera, H.L. Bardet-Biedl syndrome proteins modulate the release of bioactive extracellular vesicles. Nat. Commun. 2021, 12, 5671. [Google Scholar] [CrossRef] [PubMed]
- Mohieldin, A.M.; Pala, R.; Sherpa, R.T.; Alanazi, M.; Alanazi, A.; Shamloo, K.; Ahsan, A.; AbouAlaiwi, W.A.; Moresco, J.J.; Yates, J.R., 3rd; et al. Proteomic identification reveals the role of ciliary extracellular-like vesicle in cardiovascular function. Adv. Sci. 2020, 7, 1903140. [Google Scholar] [CrossRef] [PubMed]
- Menting, M.E.; Cuypers, J.A.; Opić, P.; Utens, E.M.; Witsenburg, M.; van den Bosch, A.E.; van Domburg, R.T.; Meijboom, F.J.; Boersma, E.; Bogers, A.J.; et al. The unnatural history of the ventricular septal defect: Outcome up to 40 years after surgical closure. J. Am. Coll. Cardiol. 2015, 65, 1941–1951. [Google Scholar] [CrossRef] [PubMed]

| Ciliopathy Syndrome | Associated Genes |
|---|---|
| Alström syndrome [44] | ALMS1 |
| Bardet—Biedl syndrome [30] | BBS1-16 |
| Ellis-van Creveld syndrome [45] | EVC/EVC1, EVC2 |
| Jeune syndrome (Asphyxiating thoracic dystrophy) [46] | IFT80 |
| Joubert syndrome [47] | CEP290, others |
| Leber Congenital Amaurosis [48] | GUCY2D, RPE65, others |
| McKusick—Kaufman syndrome [49] | MKKS/BBS6 |
| Meckel—Gruber syndrome [50] | MKS1-13, others |
| Nephronophthisis [51] | NPHP1-NPHP11, others |
| Orofaciodigital syndrome 1 [42] | OFD1 |
| Polycystic Kidney Disease [21] | PKD1, PKD2 |
| Senior—Løken syndromes [52] | NPHP1, NPHP3, others |
| Sensenbrenner syndrome (Cranioectodermal dysplasia) [53] | IFT122, WDR35 |
| Short-rib polydactyly syndrome [54] | DYNC2H1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hale, Z.E.; Sadoshima, J. Primary Cilia and Their Role in Acquired Heart Disease. Cells 2022, 11, 960. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11060960
Hale ZE, Sadoshima J. Primary Cilia and Their Role in Acquired Heart Disease. Cells. 2022; 11(6):960. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11060960
Chicago/Turabian StyleHale, Zachariah E., and Junichi Sadoshima. 2022. "Primary Cilia and Their Role in Acquired Heart Disease" Cells 11, no. 6: 960. https://0-doi-org.brum.beds.ac.uk/10.3390/cells11060960