Dual Effects of miR-181b-2-3p/SOX21 Interaction on Microglia and Neural Stem Cells after Gamma Irradiation
Abstract
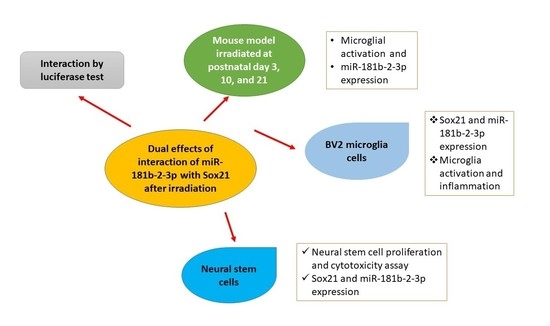
:1. Introduction
2. Materials and Methods
2.1. Animal Models
2.2. Immunohistochemical Staining
2.3. Mouse Brain RNA Extraction
2.4. Real-Time RT-PCR Analysis for miR-181b-2-3p
2.5. Predication of miR-181b-2-3p Targets and Luciferase Reporter Assay
2.6. Culture of BV2 and Neural Stem Cells (NSCs)
2.7. Western Blot
2.8. RNA Extraction from Cells and Real-Time RT-PCR Analysis for miRNA and mRNA
2.9. Knock-Down Gene Expression of SOX21 by SOX21 siRNA in BV2
2.10. TNF-a in BV2 by Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Cell Proliferation and Cytotoxicity Assay in NSCs
2.12. Overexpression of SOX21 in NSCs
2.13. Statistical Analysis
3. Results
3.1. Exposure to γ-Radiation Induced Microglial Activation and Proliferation in the Mouse Dentate Gyrus
3.2. SOX21 Was Highly Expressed in Microglia as the Target of miR-181b-2-3p
3.3. Exposure to γ-Irradiation Significantly Decreased miR-181b-2-3p Expression and Increased the mRNA and Protein Levels of SOX21 in BV2
3.4. The Knockdown of SOX21 by SOX21 siRNA Blocked the Activation of Microglia Induced by γ-Irradiation
3.5. Exposure to γ-Irradiation Caused Cytotoxicity in NSCs and Induced the Impairment of Cell Proliferation
3.6. Exposure to γ-Irradiation Increased the Expression of miR-181b-2-3p and Decreased the mRNA and Protein Expression of SOX21 in NSC
3.7. The Overexpression of SOX21 Blocked the Decreased Cell Viability Induced by γ-Irradiation in NSCs
4. Discussion
4.1. Irradiation-Induced Microglial Reaction Was Primarily Regulated by the Downregulation of miR-181b-2-3p with Upregulated SOX21
4.2. The Irradiation-Induced Impairment of Neurogenesis Was Regulated by the Upregulated miR-181b-2-3p with Downregulated SOX21
4.3. Study Limitations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minnier, J.; Emmett, M.R.; Perez, R.; Ding, L.H.; Barnette, B.L.; Larios, R.E.; Hong, C.; Hwang, T.H.; Yu, Y.; Fallgren, C.M.; et al. Associations between lipids in selected brain regions, plasma miRNA, and behavioral and cognitive measures following (28)Si ion irradiation. Sci. Rep. 2021, 11, 14899. [Google Scholar] [CrossRef]
- Segaran, R.C.; Chan, L.Y.; Wang, H.; Sethi, G.; Tang, F.R. Neuronal Development-Related miRNAs as Biomarkers for Alzheimer’s Disease, Depression, Schizophrenia and Ionizing Radiation Exposure. Curr. Med. Chem. 2021, 28, 19–52. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Z.; Shen, H.; Wu, Z.; Liu, L.; Ren, B.; Wong, P.; Sethi, G.; Tang, F. Early Life Irradiation-Induced Hypoplasia and Impairment of Neurogenesis in the Dentate Gyrus and Adult Depression Are Mediated by MicroRNA- 34a-5p/T-Cell Intracytoplasmic Antigen-1 Pathway. Cells 2021, 10, 2476. [Google Scholar] [CrossRef]
- Hutchison, E.R.; Kawamoto, E.M.; Taub, D.D.; Lal, A.; Abdelmohsen, K.; Zhang, Y.; Wood, W.H., 3rd; Lehrmann, E.; Camandola, S.; Becker, K.G.; et al. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia 2013, 61, 1018–1028. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, J.; Xu, C.; Wang, Y.; Sun, L.; Guo, X.; Liu, H. MicroRNA-181 regulates CARM1 and histone arginine methylation to promote differentiation of human embryonic stem cells. PloS ONE 2013, 8, e53146. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Zhao, H.; Tao, Z.; Wang, R.; Liu, P.; Han, Z.; Ma, S.; Luo, Y.; Jia, J. MicroRNA-181c Exacerbates Brain Injury in Acute Ischemic Stroke. Aging Dis. 2016, 7, 705–714. [Google Scholar] [CrossRef] [Green Version]
- Jeyaseelan, K.; Lim, K.Y.; Armugam, A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 2008, 39, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Y.B.; Lu, Y.; Yue, S.; Xu, L.J.; Xiong, X.X.; White, R.E.; Sun, X.; Giffard, R.G. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol. Dis. 2012, 45, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.M.; Xu, L.; Giffard, R.G. Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J. Cereb. Blood Flow Metab. 2013, 33, 1976–1982. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Dong, L.Y.; Li, Y.J.; Hong, Z.; Wei, W.S. The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor. J. Neuroinflammation 2012, 9, 211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, Y.J.; Wu, X.Y.; Hong, Z.; Wei, W.S. MicroRNA-181c negatively regulates the inflammatory response in oxygen-glucose-deprived microglia by targeting Toll-like receptor 4. J. Neurochem. 2015, 132, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; He, X.; Lu, F.; Mao, H.; Zhu, Z.; Yao, L.; Luo, W.; Sun, X.; Wang, B.; Qian, C.; et al. A lincRNA-p21/miR-181 family feedback loop regulates microglial activation during systemic LPS- and MPTP- induced neuroinflammation. Cell Death Dis. 2018, 9, 803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhry, M.A.; Omaruddin, R.A.; Brumbaugh, C.D.; Tariq, M.A.; Pourmand, N. Identification of radiation-induced microRNA transcriptome by next-generation massively parallel sequencing. J. Radiat. Res. 2013, 54, 808–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Icli, B.; Wara, A.K.; Belkin, N.; He, S.; Kobzik, L.; Hunninghake, G.M.; Vera, M.P.; Blackwell, T.S.; Baron, R.M.; et al. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J. Clin. Investig. 2012, 122, 1973–1990. [Google Scholar] [CrossRef] [PubMed]
- Tektemur, A.; Tektemur, N.K.; Güzel, E.E. The therapeutic effect of hesperetin on doxorubicin-induced testicular toxicity: Potential roles of the mechanistic target of rapamycin kinase (mTOR) and dynamin-related protein 1 (DRP1). Toxicol. Appl. Pharmacol. 2022, 435, 115833. [Google Scholar] [CrossRef]
- Zeng, C.; Fan, D.; Xu, Y.; Li, X.; Yuan, J.; Yang, Q.; Zhou, X.; Lu, J.; Zhang, C.; Han, J.; et al. Curcumol enhances the sensitivity of doxorubicin in triple-negative breast cancer via regulating the miR-181b-2-3p-ABCC3 axis. Biochem. Pharmacol. 2020, 174, 113795. [Google Scholar] [CrossRef]
- Bylund, M.; Andersson, E.; Novitch, B.G.; Muhr, J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 2003, 6, 1162–1168. [Google Scholar] [CrossRef]
- Graham, V.; Khudyakov, J.; Ellis, P.; Pevny, L. SOX2 functions to maintain neural progenitor identity. Neuron 2003, 39, 749–765. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, M.; Kallstrom, M.; Muhr, J. Sox21 promotes the progression of vertebrate neurogenesis. Nat. Neurosci. 2005, 8, 995–1001. [Google Scholar] [CrossRef]
- Matsuda, S.; Kuwako, K.; Okano, H.J.; Tsutsumi, S.; Aburatani, H.; Saga, Y.; Matsuzaki, Y.; Akaike, A.; Sugimoto, H.; Okano, H. Sox21 promotes hippocampal adult neurogenesis via the transcriptional repression of the Hes5 gene. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 12543–12557. [Google Scholar] [CrossRef] [Green Version]
- Kozareva, D.A.; Moloney, G.M.; Hoban, A.E.; Rossini, V.; Nally, K.; Cryan, J.F.; Nolan, Y.M. A role for the orphan nuclear receptor TLX in the interaction between neural precursor cells and microglia. Health Psychol. Behav. Med. 2019, 3, Ns20180177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadhwa, M.; Prabhakar, A.; Ray, K.; Roy, K.; Kumari, P.; Jha, P.K.; Kishore, K.; Kumar, S.; Panjwani, U. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J. Neuroinflammation 2017, 14, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Liu, T.; Huang, W.; Liu, H.; Zhang, H.M.; Li, Q.; Chen, Z.; Guo, A.Y. MicroRNA regulatory pathway analysis identifies miR-142-5p as a negative regulator of TGF-beta pathway via targeting SMAD3. Oncotarget 2016, 7, 71504–71513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Dong, J.H.; Huang, G.D.; Qu, X.F.; Wu, G.; Dong, X.R. NF-kappaB signaling modulates radiationinduced microglial activation. Oncol. Rep. 2014, 31, 2555–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monje, M.L.; Mizumatsu, S.; Fike, J.R.; Palmer, T.D. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 2002, 8, 955–962. [Google Scholar] [CrossRef]
- Betlazar, C.; Middleton, R.J.; Howell, N.; Storer, B.; Davis, E.; Davies, J.; Banati, R.; Liu, G.J. Mitochondrial Translocator Protein (TSPO) Expression in the Brain after Whole Body Gamma Irradiation. Front. Cell Dev. Biol. 2021, 9, 715444. [Google Scholar] [CrossRef]
- Lee, W.H.; Sonntag, W.E.; Mitschelen, M.; Yan, H.; Lee, Y.W. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int. J. Radiat. Biol. 2010, 86, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef]
- Chen, H.; Chong, Z.Z.; De Toledo, S.M.; Azzam, E.I.; Elkabes, S.; Souayah, N. Delayed activation of human microglial cells by high dose ionizing radiation. Brain Res. 2016, 1646, 193–198. [Google Scholar] [CrossRef]
- Ren, B.X.; Huen, I.; Wu, Z.J.; Wang, H.; Duan, M.Y.; Guenther, I.; Bhanu Prakash, K.N.; Tang, F.R. Early postnatal irradiation-induced age-dependent changes in adult mouse brain: MRI based characterization. BMC Neurosci. 2021, 22, 28. [Google Scholar] [CrossRef]
- Leavitt, R.J.; Acharya, M.M.; Baulch, J.E.; Limoli, C.L. Extracellular Vesicle-Derived miR-124 Resolves Radiation-Induced Brain Injury. Cancer Res. 2020, 80, 4266–4277. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Fan, W.; Sun, F.; Li, M.; Lin, M.; Yu, Y.; Liang, S.; Liao, H.; Jie, W.; Cai, Y.; et al. Nasal Delivery of AntagomiR-741 Protects Against the Radiation-Induced Brain Injury in Mice. Radiat. Res. 2021, 195, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Collignon, J.; Sockanathan, S.; Hacker, A.; Cohen-Tannoudji, M.; Norris, D.; Rastan, S.; Stevanovic, M.; Goodfellow, P.N.; Lovell-Badge, R. A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 1996, 122, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.; Cheah, P.S.; Szarek, E.; Banerjee, K.; Schwartz, J.; Thomas, P. Expression of the murine transcription factor SOX3 during embryonic and adult neurogenesis. Gene Expr. Patterns GEP 2013, 13, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.; Israsena, N.; Zhang, Z.; Hu, M.; Zhao, L.R.; Jalali, A.; Sahni, V.; Kessler, J.A. Sox1 acts through multiple independent pathways to promote neurogenesis. Dev. Biol. 2004, 269, 580–594. [Google Scholar] [CrossRef] [Green Version]
- Cimadamore, F.; Amador-Arjona, A.; Chen, C.; Huang, C.T.; Terskikh, A.V. SOX2-LIN28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc. Natl. Acad. Sci. USA 2013, 110, E3017–E3026. [Google Scholar] [CrossRef] [Green Version]
- Zaletel, I.; Schwirtlich, M.; Perović, M.; Jovanović, M.; Stevanović, M.; Kanazir, S.; Puškaš, N. Early Impairments of Hippocampal Neurogenesis in 5xFAD Mouse Model of Alzheimer’s Disease are Associated with Altered Expression of SOXB Transcription Factors. J. Alzheimer’s Dis. JAD 2018, 65, 963–976. [Google Scholar] [CrossRef]
- Abdi, S.; Javanmehr, N.; Ghasemi-Kasman, M.; Bali, H.Y.; Pirzadeh, M. Stem Cell-based Therapeutic and Diagnostic Approaches in Alzheimer’s Disease. Curr. Neuropharmacol. 2021, 20, 1093–1115. [Google Scholar] [CrossRef]
- Mizuno, Y.; Abolhassani, N.; Mazzei, G.; Sakumi, K.; Saito, T.; Saido, T.C.; Ninomiya, T.; Iwaki, T.; Yamasaki, R.; Kira, J.I.; et al. Mutyh Actively Contributes to Microglial Activation and Impaired Neurogenesis in the Pathogenesis of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 8635088. [Google Scholar] [CrossRef]
- Whittington, N.; Cunningham, D.; Le, T.K.; De Maria, D.; Silva, E.M. Sox21 regulates the progression of neuronal differentiation in a dose-dependent manner. Dev. Biol. 2015, 397, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Capilla-Gonzalez, V.; Guerrero-Cazares, H.; Bonsu, J.M.; Gonzalez-Perez, O.; Achanta, P.; Wong, J.; Garcia-Verdugo, J.M.; Quiñones-Hinojosa, A. The subventricular zone is able to respond to a demyelinating lesion after localized radiation. Stem Cells 2014, 32, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arango-Gonzalez, B.; Schatz, A.; Bolz, S.; Eslava-Schmalbach, J.; Willmann, G.; Zhour, A.; Zrenner, E.; Fischer, M.D.; Gekeler, F. Effects of combined ketamine/xylazine anesthesia on light induced retinal degeneration in rats. PloS ONE 2012, 7, e35687. [Google Scholar] [CrossRef]
- Ferro, M.M.; Angelucci, M.E.; Anselmo-Franci, J.A.; Canteras, N.S.; Da Cunha, C. Neuroprotective effect of ketamine/xylazine on two rat models of Parkinson’s disease. Braz. J. Med. Biol. Res. 2007, 40, 89–96. [Google Scholar] [CrossRef]
- Balentova, S.; Adamkov, M. Molecular, Cellular and Functional Effects of Radiation-Induced Brain Injury: A Review. Int. J. Mol. Sci. 2015, 16, 27796–27815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balentova, S.; Hajtmanova, E.; Adamkov, M.; Lehotsky, J. Differential expression of doublecortin and microglial markers in the rat brain following fractionated irradiation. Neurochem. Res. 2015, 40, 501–513. [Google Scholar] [CrossRef]
- Kroehl, M.E.; Lutz, S.; Wagner, B.D. Permutation-based methods for mediation analysis in studies with small sample sizes. Peer J. 2020, 8, e8246. [Google Scholar] [CrossRef]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ma, Z.-W.; Ho, F.-M.; Sethi, G.; Tang, F.R. Dual Effects of miR-181b-2-3p/SOX21 Interaction on Microglia and Neural Stem Cells after Gamma Irradiation. Cells 2023, 12, 649. https://0-doi-org.brum.beds.ac.uk/10.3390/cells12040649
Wang H, Ma Z-W, Ho F-M, Sethi G, Tang FR. Dual Effects of miR-181b-2-3p/SOX21 Interaction on Microglia and Neural Stem Cells after Gamma Irradiation. Cells. 2023; 12(4):649. https://0-doi-org.brum.beds.ac.uk/10.3390/cells12040649
Chicago/Turabian StyleWang, Hong, Zhao-Wu Ma, Feng-Ming Ho, Gautam Sethi, and Feng Ru Tang. 2023. "Dual Effects of miR-181b-2-3p/SOX21 Interaction on Microglia and Neural Stem Cells after Gamma Irradiation" Cells 12, no. 4: 649. https://0-doi-org.brum.beds.ac.uk/10.3390/cells12040649