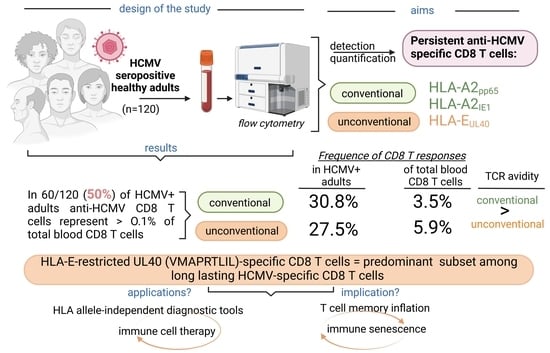
Persistent CD8 T Cell Marks Caused by the HCMV Infection in Seropositive Adults: Prevalence of HLA-E-Reactive CD8 T Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Samples and PBMC Isolation
2.2. MHC/Peptide Tetramer Complexes
2.3. Flow Cytometry Protocol for the Detection and Quantification of HCMV-Specific CD8 T Cell Populations
2.4. Data Analysis and Statistics
3. Results
3.1. Frequency and Pattern of HCMV-Specific CD8 T Cell Responses in HCMV+ Healthy Adults
3.2. Relative Frequencies of HCMV Peptide-Specific CD8 T Cell Responses among Total Blood CD8 T Cells and Impact on Host T Cell Homeostasis
3.3. Long-Lasting HCMV-Specific CD8 T Cells in Healthy Adults: TCR Avidity and Co-Receptor Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Broers, A.E.; van der Holt, R.; van Esser, J.W.; Gratama, J.W.; Henzen-Logmans, S.; Kuenen-Boumeester, V.; Löwenberg, B.; Cornelissen, J.J. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood 2000, 95, 2240–2245. [Google Scholar] [CrossRef] [Green Version]
- Adland, E.; Klenerman, P.; Goulder, P.; Matthews, P.C. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Front. Microbiol. 2015, 6, 1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaminski, H.; Fishman, J.A. The Cell Biology of Cytomegalovirus: Implications for Transplantation. Am. J. Transplant. 2016, 16, 2254–2269. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Navti, O.B.; Al-Belushi, M.; Konje, J.C.; FRCOG. Cytomegalovirus infection in pregnancy—An update. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Shiohara, T. Current understanding of cytomegalovirus infection in immunocompetent individuals. J. Dermatol. Sci. 2000, 22, 196–204. [Google Scholar] [CrossRef]
- Chiereghin, A.; Verucchi, G.; Lazzarotto, T. CMV-Specific Cell-Mediated Immunity in Immunocompetent Adults with Primary CMV Infection: A Case Series and Review of the Literature. Viruses 2021, 13, 816. [Google Scholar] [CrossRef]
- van den Berg, S.P.H.; Pardieck, I.N.; Lanfermeijer, J.; Sauce, D.; Klenerman, P.; van Baarle, D.; Arens, R. The hallmarks of CMV-specific CD8 T-cell differentiation. Med. Microbiol. Immunol. 2019, 208, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Klenerman, P.; Oxenius, A. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 2016, 16, 367–377. [Google Scholar] [CrossRef]
- Lopez-Verges, S.; Milush, J.M.; Schwartz, B.S.; Pando, M.J.; Jarjoura, J.; York, V.A.; Houchins, J.P.; Miller, S.; Kang, S.M.; Norris, P.J.; et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 14725–14732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karrer, U.; Sierro, S.; Wagner, M.; Oxenius, A.; Hengel, H.; Koszinowski, U.H.; Phillips, R.E.; Klenerman, P. Memory inflation: Continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 2003, 170, 2022–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadrup, S.R.; Strindhall, J.; Kollgaard, T.; Seremet, T.; Johansson, B.; Pawelec, G.; thor Straten, P.; Wikby, A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 2006, 176, 2645–2653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawelec, G.; Derhovanessian, E. Role of CMV in immune senescence. Virus Res. 2011, 157, 175–179. [Google Scholar] [CrossRef]
- Sansoni, P.; Vescovini, R.; Fagnoni, F.F.; Akbar, A.; Arens, R.; Chiu, Y.L.; Cicin-Sain, L.; Dechanet-Merville, J.; Derhovanessian, E.; Ferrando-Martinez, S.; et al. New advances in CMV and immunosenescence. Exp. Gerontol. 2014, 55, 54–62. [Google Scholar] [CrossRef]
- Savva, G.M.; Pachnio, A.; Kaul, B.; Morgan, K.; Huppert, F.A.; Brayne, C.; Moss, P.A.; Medical Research Council Cognitive, F.; Ageing, S. Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell 2013, 12, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Firth, C.; Harrison, R.; Ritchie, S.; Wardlaw, J.; Ferro, C.J.; Starr, J.M.; Deary, I.J.; Moss, P. Cytomegalovirus infection is associated with an increase in systolic blood pressure in older individuals. QJM 2016, 109, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Doerr, H.W.; Cinatl, J. The story of human cytomegalovirus and cancer: Increasing evidence and open questions. Neoplasia 2009, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kern, F.; Surel, I.P.; Faulhaber, N.; Frommel, C.; Schneider-Mergener, J.; Schonemann, C.; Reinke, P.; Volk, H.D. Target structures of the CD8(+)-T-cell response to human cytomegalovirus: The 72-kilodalton major immediate-early protein revisited. J. Virol. 1999, 73, 8179–8184. [Google Scholar] [CrossRef] [Green Version]
- Weekes, M.P.; Wills, M.R.; Mynard, K.; Carmichael, A.J.; Sissons, J.G. The memory cytotoxic T-lymphocyte (CTL) response to human cytomegalovirus infection contains individual peptide-specific CTL clones that have undergone extensive expansion in vivo. J. Virol. 1999, 73, 2099–2108. [Google Scholar] [CrossRef] [Green Version]
- Sylwester, A.W.; Mitchell, B.L.; Edgar, J.B.; Taormina, C.; Pelte, C.; Ruchti, F.; Sleath, P.R.; Grabstein, K.H.; Hosken, N.A.; Kern, F.; et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005, 202, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Sylwester, A.; Nambiar, K.Z.; Caserta, S.; Klenerman, P.; Picker, L.J.; Kern, F. A new perspective of the structural complexity of HCMV-specific T-cell responses. Mech. Ageing Dev. 2016, 158, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Soderstrom, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef]
- Petrie, E.J.; Clements, C.S.; Lin, J.; Sullivan, L.C.; Johnson, D.; Huyton, T.; Heroux, A.; Hoare, H.L.; Beddoe, T.; Reid, H.H.; et al. CD94-NKG2A recognition of human leukocyte antigen (HLA)-E bound to an HLA class I leader sequence. J. Exp. Med. 2008, 205, 725–735. [Google Scholar] [CrossRef] [Green Version]
- Pietra, G.; Romagnani, C.; Falco, M.; Vitale, M.; Castriconi, R.; Pende, D.; Millo, E.; Anfossi, S.; Biassoni, R.; Moretta, L.; et al. The analysis of the natural killer-like activity of human cytolytic T lymphocytes revealed HLA-E as a novel target for TCR alpha/beta-mediated recognition. Eur. J. Immunol. 2001, 31, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, C.; Pietra, G.; Falco, M.; Millo, E.; Mazzarino, P.; Biassoni, R.; Moretta, A.; Moretta, L.; Mingari, M.C. Identification of HLA-E-specific alloreactive T lymphocytes: A cell subset that undergoes preferential expansion in mixed lymphocyte culture and displays a broad cytolytic activity against allogeneic cells. Proc. Natl. Acad. Sci. USA 2002, 99, 11328–11333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoare, H.L.; Sullivan, L.C.; Pietra, G.; Clements, C.S.; Lee, E.J.; Ely, L.K.; Beddoe, T.; Falco, M.; Kjer-Nielsen, L.; Reid, H.H.; et al. Structural basis for a major histocompatibility complex class Ib-restricted T cell response. Nat. Immunol. 2006, 7, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.; Tonnerre, P.; Nedellec, S.; Oger, R.; Morice, A.; Guilloux, Y.; Houssaint, E.; Charreau, B.; Gervois, N. HLA-E-restricted cross-recognition of allogeneic endothelial cells by CMV-associated CD8 T cells: A potential risk factor following transplantation. PLoS ONE 2012, 7, e50951. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, L.C.; Westall, G.P.; Widjaja, J.M.; Mifsud, N.A.; Nguyen, T.H.; Meehan, A.C.; Kotsimbos, T.C.; Brooks, A.G. The Presence of HLA-E-Restricted, CMV-Specific CD8+ T Cells in the Blood of Lung Transplant Recipients Correlates with Chronic Allograft Rejection. PLoS ONE 2015, 10, e0135972. [Google Scholar] [CrossRef] [Green Version]
- Jouand, N.; Bressollette-Bodin, C.; Gerard, N.; Giral, M.; Guerif, P.; Rodallec, A.; Oger, R.; Parrot, T.; Allard, M.; Cesbron-Gautier, A.; et al. HCMV triggers frequent and persistent UL40-specific unconventional HLA-E-restricted CD8 T-cell responses with potential autologous and allogeneic peptide recognition. PLoS Pathog. 2018, 14, e1007041. [Google Scholar] [CrossRef]
- Rousseliere, A.; Gerard, N.; Delbos, L.; Guerif, P.; Giral, M.; Bressollette-Bodin, C.; Charreau, B. Distinctive phenotype for HLA-E- versus HLA-A2-restricted memory CD8 alphabetaT cells in the course of HCMV infection discloses features shared with NKG2C(+)CD57(+)NK and delta2(-)gammadeltaT cell subsets. Front. Immunol. 2022, 13, 1063690. [Google Scholar] [CrossRef]
- Garrigue, I.; Faure-Della Corte, M.; Magnin, N.; Recordon-Pinson, P.; Couzi, L.; Lebrette, M.E.; Schrive, M.H.; Roncin, L.; Taupin, J.L.; Dechanet-Merville, J.; et al. UL40 human cytomegalovirus variability evolution patterns over time in renal transplant recipients. Transplantation 2008, 86, 826–835. [Google Scholar] [CrossRef]
- Heatley, S.L.; Pietra, G.; Lin, J.; Widjaja, J.M.; Harpur, C.M.; Lester, S.; Rossjohn, J.; Szer, J.; Schwarer, A.; Bradstock, K.; et al. Polymorphism in human cytomegalovirus UL40 impacts on recognition of human leukocyte antigen-E (HLA-E) by natural killer cells. J. Biol. Chem. 2013, 288, 8679–8690. [Google Scholar] [CrossRef] [Green Version]
- Vietzen, H.; Ruckert, T.; Hartenberger, S.; Honsig, C.; Jaksch, P.; Geleff, S.; Hammer, Q.; Romagnani, C.; Segura-Wang, M.; Puchhammer-Stockl, E. Extent of Cytomegalovirus Replication in the Human Host Depends on Variations of the HLA-E/UL40 Axis. mBio 2021, 12, e02996-20. [Google Scholar] [CrossRef]
- Bodinier, M.; Peyrat, M.A.; Tournay, C.; Davodeau, F.; Romagne, F.; Bonneville, M.; Lang, F. Efficient detection and immunomagnetic sorting of specific T cells using multimers of MHC class I and peptide with reduced CD8 binding. Nat. Med. 2000, 6, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Colugnati, F.A.; Staras, S.A.; Dollard, S.C.; Cannon, M.J. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect. Dis. 2007, 7, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, S.; Malecek, K.; Johnson, L.A.; Yu, Z.; Vega-Saenz de Miera, E.; Darvishian, F.; McGary, K.; Huang, K.; Boyer, J.; Corse, E.; et al. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc. Natl. Acad. Sci. USA 2013, 110, 6973–6978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisler, J.; Touma, J.; Rahbar, A.; Soderberg-Naucler, C.; Vetvik, K. A Review of the Potential Role of Human Cytomegalovirus (HCMV) Infections in Breast Cancer Carcinogenesis and Abnormal Immunity. Cancers 2019, 11, 1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braud, V.; Jones, E.Y.; McMichael, A. The human major histocompatibility complex class Ib molecule HLA-E binds signal sequence-derived peptides with primary anchor residues at positions 2 and 9. Eur. J. Immunol. 1997, 27, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Jabri, B.; Selby, J.M.; Negulescu, H.; Lee, L.; Roberts, A.I.; Beavis, A.; Lopez-Botet, M.; Ebert, E.C.; Winchester, R.J. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity 2002, 17, 487–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarrago, D.; Gonzalez, I.; Gonzalez-Escribano, M.F. HLA-E restricted cytomegalovirus UL40 peptide polymorphism may represent a risk factor following congenital infection. BMC Genom. 2022, 23, 455. [Google Scholar] [CrossRef]
- Wooldridge, L.; Lissina, A.; Vernazza, J.; Gostick, E.; Laugel, B.; Hutchinson, S.L.; Mirza, F.; Dunbar, P.R.; Boulter, J.M.; Glick, M.; et al. Enhanced immunogenicity of CTL antigens through mutation of the CD8 binding MHC class I invariant region. Eur. J. Immunol. 2007, 37, 1323–1333. [Google Scholar] [CrossRef]
- Gao, G.F.; Willcox, B.E.; Wyer, J.R.; Boulter, J.M.; O’Callaghan, C.A.; Maenaka, K.; Stuart, D.I.; Jones, E.Y.; Van Der Merwe, P.A.; Bell, J.I.; et al. Classical and nonclassical class I major histocompatibility complex molecules exhibit subtle conformational differences that affect binding to CD8alphaalpha. J. Biol. Chem. 2000, 275, 15232–15238. [Google Scholar] [CrossRef] [Green Version]
- Rius, C.; Attaf, M.; Tungatt, K.; Bianchi, V.; Legut, M.; Bovay, A.; Donia, M.; Thor Straten, P.; Peakman, M.; Svane, I.M.; et al. Peptide-MHC Class I Tetramers Can Fail To Detect Relevant Functional T Cell Clonotypes and Underestimate Antigen-Reactive T Cell Populations. J. Immunol. 2018, 200, 2263–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle-Arroyo, J.; Aguado, R.; Paez-Vega, A.; Perez, A.B.; Gonzalez, R.; Fornes, G.; Torre-Cisneros, J.; Cantisan, S. Lack of cytomegalovirus (CMV)-specific cell-mediated immune response using QuantiFERON-CMV assay in CMV-seropositive healthy volunteers: Fact not artifact. Sci. Rep. 2020, 10, 7194. [Google Scholar] [CrossRef]
- Strong, R.K.; Holmes, M.A.; Li, P.; Braun, L.; Lee, N.; Geraghty, D.E. HLA-E allelic variants. Correlating differential expression, peptide affinities, crystal structures, and thermal stabilities. J. Biol. Chem. 2003, 278, 5082–5090. [Google Scholar] [CrossRef] [Green Version]
- Neill, L.; Peggs, K. Cell therapy for cytomegalovirus infection. Expert Opin. Biol. Ther. 2021, 21, 649–659. [Google Scholar] [CrossRef]
- Coupel, S.; Moreau, A.; Hamidou, M.; Horejsi, V.; Soulillou, J.P.; Charreau, B. Expression and release of soluble HLA-E is an immunoregulatory feature of endothelial cell activation. Blood 2007, 109, 2806–2814. [Google Scholar] [CrossRef] [Green Version]
- Pietra, G.; Romagnani, C.; Manzini, C.; Moretta, L.; Mingari, M.C. The emerging role of HLA-E-restricted CD8+ T lymphocytes in the adaptive immune response to pathogens and tumors. J. Biomed. Biotechnol. 2010, 2010, 907092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sottile, R.; Panjwani, M.K.; Lau, C.M.; Daniyan, A.F.; Tanaka, K.; Barker, J.N.; Brentjens, R.J.; Sun, J.C.; Le Luduec, J.B.; Hsu, K.C. Human cytomegalovirus expands a CD8(+) T cell population with loss of BCL11B expression and gain of NK cell identity. Sci. Immunol. 2021, 6, eabe6968. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousselière, A.; Charreau, B. Persistent CD8 T Cell Marks Caused by the HCMV Infection in Seropositive Adults: Prevalence of HLA-E-Reactive CD8 T Cells. Cells 2023, 12, 889. https://0-doi-org.brum.beds.ac.uk/10.3390/cells12060889
Rousselière A, Charreau B. Persistent CD8 T Cell Marks Caused by the HCMV Infection in Seropositive Adults: Prevalence of HLA-E-Reactive CD8 T Cells. Cells. 2023; 12(6):889. https://0-doi-org.brum.beds.ac.uk/10.3390/cells12060889
Chicago/Turabian StyleRousselière, Amélie, and Béatrice Charreau. 2023. "Persistent CD8 T Cell Marks Caused by the HCMV Infection in Seropositive Adults: Prevalence of HLA-E-Reactive CD8 T Cells" Cells 12, no. 6: 889. https://0-doi-org.brum.beds.ac.uk/10.3390/cells12060889