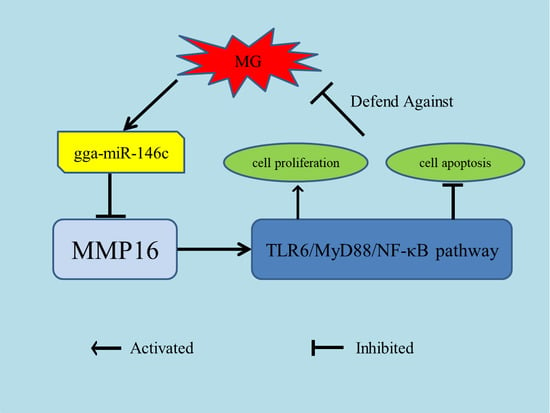
gga-miR-146c Activates TLR6/MyD88/NF-κB Pathway through Targeting MMP16 to Prevent Mycoplasma Gallisepticum (HS Strain) Infection in Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Declaration
2.2. Mycoplasma Strains
2.3. Cell infection Experiments
2.4. gga-miR-146c Target Gene Prediction
2.5. RNA Oligonucleotides and DNA Primers
2.6. Dual-Luciferase Reporter Assay
2.7. RNA Extraction and RT-qPCR
2.8. Immunofluorescence
2.9. ELISA
2.10. Western-Blot Analysis
2.11. Cell Proliferation, Cycle, and Apoptosis
2.12. Statistical Analysis
3. Results
3.1. gga-miR-146c Expression Was Upregulated after MG Infection
3.2. gga-miR-146c Contains Conserved Target Site of MMP16
3.3. MMP16 Is a Direct Target Gene of gga-miR-146c
3.4. MG Infection Downregulates MMP16 Expression
3.5. gga-miR-146c Is of Importance in NF-κB Pathway Regulation
3.6. Upregulation of gga-miR-146c Promotes Proliferation and Cycle Progression by Repressing Apoptosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gambarini, M.L.; Kunz, T.L.; Oliveira Filho, B.D.; Porto, R.N.; Oliveira, C.M.; Brito, W.M.; Viu, M.A. Granular Vulvovaginitis Syndrome in Nelore pubertal and post pubertal replacement heifers under tropical conditions: role of Mycoplasma spp., Ureaplasma diversum and BHV-1. Trop. Anim. Health Prod. 2009, 41, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R.; Ayling, R. Mycoplasma in cattle. Vet. Rec. 2016, 178, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E. Feline hemotropic mycoplasmas. J. Vet. Emerg. Crit. Care (San Antonio) 2010, 20, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.R.; Nettles, V.F.; Couvillion, C.E.; Yoder, H.W., Jr. Infectious sinusitis in wild turkeys. Avian Dis. 1982, 26, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Stipkovits, L.; Egyed, L.; Palfi, V.; Beres, A.; Pitlik, E.; Somogyi, M.; Szathmary, S.; Denes, B. Effect of low-pathogenicity influenza virus H3N8 infection on Mycoplasma gallisepticum infection of chickens. Avian Pathol. 2012, 41, 51–57. [Google Scholar] [CrossRef]
- Quirk, M. Antibiotic-resistant bacteria in food animals on the rise. Lancet Infect. Dis. 2001, 1, 293. [Google Scholar] [CrossRef]
- Hofacre, C.L.; White, D.G.; Maurer, J.J.; Morales, C.; Lobsinger, C.; Hudson, C. Characterization of antibiotic-resistant bacteria in rendered animal products. Avian Dis. 2001, 45, 953–961. [Google Scholar] [CrossRef]
- Witter, R.L. Control strategies for Marek’s disease: a perspective for the future. Poult. Sci. 1998, 77, 1197–1203. [Google Scholar] [CrossRef]
- Pennycott, T.W.; Dare, C.M.; Yavari, C.A.; Bradbury, J.M. Mycoplasma sturni and Mycoplasma gallisepticum in wild birds in Scotland. Vet. Rec. 2005, 156, 513–515. [Google Scholar] [CrossRef]
- O’Reilly, S. MicroRNAs in fibrosis: opportunities and challenges. Arthritis Res. Ther. 2016, 18, 11. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Rao, D.S.; Chaudhuri, A.A.; Baltimore, D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 2010, 10, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Jorganes, A.; Lee, M.Y.; Araldi, E.; Landskroner-Eiger, S.; Fernandez-Fuertes, M.; Sahraei, M.; Quiles Del Rey, M.; van Solingen, C.; Yu, J.; Fernandez-Hernando, C.; et al. VEGF-Induced Expression of miR-17-92 Cluster in Endothelial Cells Is Mediated by ERK/ELK1 Activation and Regulates Angiogenesis. Circ. Res. 2016, 118, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Li, X.; Zhao, C.; Han, B.; Qu, L.; Song, J.; Liu, C.; Yang, N. Chicken gga-miR-181a targets MYBL1 and shows an inhibitory effect on proliferation of Marek’s disease virus-transformed lymphoid cell line. Poult. Sci. 2015, 94, 2616–2621. [Google Scholar] [CrossRef]
- Lin, J.; Xia, J.; Chen, Y.T.; Zhang, K.Y.; Zeng, Y.; Yang, Q. H9N2 avian influenza virus enhances the immune responses of BMDCs by down-regulating miR29c. Vaccine 2017, 35, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Shang, H.; Shu, D.; Zhang, H.; Ji, J.; Sun, B.; Li, H.; Xie, Q. gga-miR-375 plays a key role in tumorigenesis post subgroup J avian leukosis virus infection. PLoS ONE 2014, 9, e90878. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Wang, Y.S.; Meng, K.; Pan, Q.X.; Wang, X.L.; Xia, X.X.; Zhu, Y.M.; Bi, Z.W.; Zhang, H.B.; Luo, K. gga-miR-2127 downregulates the translation of chicken p53 and attenuates chp53-mediated innate immune response against IBDV infection. Vet. Microbiol. 2017, 198, 34–42. [Google Scholar] [CrossRef]
- Hu, Q.; Zhao, Y.; Wang, Z.; Hou, Y.; Bi, D.; Sun, J.; Peng, X. Chicken gga-miR-19a Targets ZMYND11 and Plays an Important Role in Host Defense against Mycoplasma gallisepticum (HS Strain) Infection. Front. Cell. Infect. Microbiol. 2016, 6, 102. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, Z.; Hou, Y.; Zhang, K.; Peng, X. gga-miR-99a targets SMARCA5 to regulate Mycoplasma gallisepticum (HS strain) infection by depressing cell proliferation in chicken. Gene 2017, 627, 239–247. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Bi, D.; Hou, Y.; Zhao, Y.; Sun, J.; Peng, X. Gga-miR-101-3p Plays a Key Role in Mycoplasma gallisepticum (HS Strain) Infection of Chicken. Int. J. Mol. Sci. 2015, 16, 28669–28682. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Yang, J.P.; Hori, M.; Sanda, T.; Okamoto, T. Identification of a novel inhibitor of nuclear factor-kappaB, RelA-associated inhibitor. J. Biol. Chem. 1999, 274, 15662–15670. [Google Scholar] [CrossRef]
- Lecellier, C.H.; Dunoyer, P.; Arar, K.; Lehmann-Che, J.; Eyquem, S.; Himber, C.; Saib, A.; Voinnet, O. A cellular microRNA mediates antiviral defense in human cells. Science 2005, 308, 557–560. [Google Scholar] [CrossRef]
- Zheng, C.Z.; Shu, Y.B.; Luo, Y.L.; Luo, J. The role of miR-146a in modulating TRAF6-induced inflammation during lupus nephritis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1041–1048. [Google Scholar]
- Jang, S.Y.; Chae, M.K.; Lee, J.H.; Lee, E.J.; Yoon, J.S. Role of miR-146a in the Regulation of Inflammation in an In Vitro Model of Graves’ Orbitopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4027–4034. [Google Scholar] [CrossRef]
- Meisgen, F.; Xu Landen, N.; Wang, A.; Rethi, B.; Bouez, C.; Zuccolo, M.; Gueniche, A.; Stahle, M.; Sonkoly, E.; Breton, L.; et al. MiR-146a negatively regulates TLR2-induced inflammatory responses in keratinocytes. J. Investig. Dermatol. 2014, 134, 1931–1940. [Google Scholar] [CrossRef]
- Liang, G.; Malmuthuge, N.; Guan, Y.; Ren, Y.; Griebel, P.J.; Guan, L.L. Altered microRNA expression and pre-mRNA splicing events reveal new mechanisms associated with early stage Mycobacterium avium subspecies paratuberculosis infection. Sci. Rep. 2016, 6, 24964. [Google Scholar] [CrossRef]
- Liu, P.; Yang, F.; Zhuang, Y.; Xiao, Q.; Cao, H.; Zhang, C.; Wang, T.; Lin, H.; Guo, X.; Hu, G. Dysregulated expression of microRNAs and mRNAs in pulmonary artery remodeling in ascites syndrome in broiler chickens. Oncotarget 2017, 8, 1993–2007. [Google Scholar] [CrossRef]
- Curtale, G.; Mirolo, M.; Renzi, T.A.; Rossato, M.; Bazzoni, F.; Locati, M. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc. Natl. Acad. Sci. USA 2013, 110, 11499–11504. [Google Scholar] [CrossRef]
- Peng, X.; Gao, Q.S.; Zhou, L.; Chen, Z.H.; Lu, S.; Huang, H.J.; Zhan, C.Y.; Xiang, M. MicroRNAs in avian influenza virus H9N2-infected and non-infected chicken embryo fibroblasts. Genet. Mol. Res. 2015, 14, 9081–9091. [Google Scholar] [CrossRef]
- Lian, L.; Qu, L.; Chen, Y.; Lamont, S.J.; Yang, N. A systematic analysis of miRNA transcriptome in Marek’s disease virus-induced lymphoma reveals novel and differentially expressed miRNAs. PLoS ONE 2012, 7, e51003. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Y.; Zhang, K.; Yuan, B.; Peng, X. Identification of differentially expressed miRNAs through high-throughput sequencing in the chicken lung in response to Mycoplasma gallisepticum HS. Comp. Biochem. Physiol. Part D Genomics Proteomics 2017, 22, 146–156. [Google Scholar] [CrossRef]
- Bi, D.; Ji, X. The isolation and identification of the mycoplasma gallisepticum. Acta Vet. Zootechnol. Sin. 1988, 1, 146–148. [Google Scholar]
- Bi, D.; Xu, Q. Study on pathogenicity of HS strain Mycoplasma gallisepticum. Chin. J. Anim. Poult. Infect. Dis. 1997, 5, 24–26. [Google Scholar]
- Calus, D.; Maes, D.; Vranckx, K.; Villareal, I.; Pasmans, F.; Haesebrouck, F. Validation of ATP luminometry for rapid and accurate titration of Mycoplasma hyopneumoniae in Friis medium and a comparison with the color changing units assay. J. Microbiol. Methods 2010, 83, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tian, W.; Zhao, C.; Hu, Q.; Sun, J.; Peng, X. Roles of Toll-like receptors 2 and 6 in the inflammatory response to Mycoplasma gallisepticum infection in DF-1 cells and in chicken embryos. Dev. Comp. Immunol. 2016, 59, 39–47. [Google Scholar] [CrossRef]
- Indikova, I.; Much, P.; Stipkovits, L.; Siebert-Gulle, K.; Szostak, M.P.; Rosengarten, R.; Citti, C. Role of the GapA and CrmA cytadhesins of Mycoplasma gallisepticum in promoting virulence and host colonization. Infect. Immun. 2013, 81, 1618–1624. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Zhao, J.; Yang, S.; Wang, L.; Cheng, G.; Liu, D.; Xiao, J.; Liu, Z.; Zhao, Z. MiRNA-29c regulates the expression of inflammatory cytokines in diabetic nephropathy by targeting tristetraprolin. Sci. Rep. 2017, 7, 2314. [Google Scholar] [CrossRef]
- Rosenberger, C.M.; Podyminogin, R.L.; Diercks, A.H.; Treuting, P.M.; Peschon, J.J.; Rodriguez, D.; Gundapuneni, M.; Weiss, M.J.; Aderem, A. miR-144 attenuates the host response to influenza virus by targeting the TRAF6-IRF7 signaling axis. PLoS Pathog. 2017, 13, e1006305. [Google Scholar] [CrossRef]
- Ji, J.; Shang, H.; Zhang, H.; Li, H.; Ma, J.; Bi, Y.; Xie, Q. Temporal changes of microRNA gga-let-7b and gga-let-7i expression in chickens challenged with subgroup J avian leukosis virus. Vet. Res. Commun. 2017. [Google Scholar] [CrossRef]
- Dai, Z.; Ji, J.; Yan, Y.; Lin, W.; Li, H.; Chen, F.; Liu, Y.; Chen, W.; Bi, Y.; Xie, Q. Role of gga-miR-221 and gga-miR-222 during Tumour Formation in Chickens Infected by Subgroup J Avian Leukosis Virus. Viruses 2015, 7, 6538–6551. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Lian, L.; Li, X.; Zhao, C.; Qu, L.; Liu, C.; Song, J.; Yang, N. Chicken gga-miR-130a targets HOXA3 and MDFIC and inhibits Marek’s disease lymphoma cell proliferation and migration. Mol. Biol. Rep. 2016, 43, 667–676. [Google Scholar] [CrossRef]
- Lian, L.; Zhang, D.; Wang, Q.; Yang, N.; Qu, L. The inhibitory effects of gga-miR-199-3p, gga-miR-140-3p, and gga-miR-221-5p in Marek’s disease tumorigenesis. Poult. Sci. 2015, 94, 2131–2135. [Google Scholar] [CrossRef]
- Khorrami, S.; Zavaran Hosseini, A.; Mowla, S.J.; Soleimani, M.; Rakhshani, N.; Malekzadeh, R. MicroRNA-146a induces immune suppression and drug-resistant colorectal cancer cells. Tumour Biol. 2017, 39, 1010428317698365. [Google Scholar] [CrossRef]
- Huang, B.Y.; Hu, P.; Zhang, D.D.; Jiang, G.M.; Liu, S.Y.; Xu, Y.; Wu, Y.F.; Xia, X.; Wang, Y. C-type natriuretic peptide suppresses mesangial proliferation and matrix expression via a MMPs/TIMPs-independent pathway in vitro. J. Recept. Signal Transduct. Res. 2017, 37, 355–364. [Google Scholar] [CrossRef]
- Mali, A.V.; Joshi, A.A.; Hegde, M.V.; Kadam Sh, S. Enterolactone Suppresses Proliferation, Migration and Metastasis of MDA-MB-231 Breast Cancer Cells Through Inhibition of uPA Induced Plasmin Activation and MMPs-Mediated ECM Remodeling. Asian Pac. J. Cancer Prev. 2017, 18, 905–915. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Chen, Y.; Xie, X.; Zhang, Y.; Ma, C.; Yang, Q.; Han, Y.; Zhu, C.; Xiong, Y.; et al. Matrix Metalloproteinase 9 Facilitates Hepatitis B Virus Replication through Binding with Type I Interferon (IFN) Receptor 1 To Repress IFN/JAK/STAT Signaling. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Rodas, P.I.; Perez, D.; Jaffret, C.; Gonzalez, Y.; Carreno, C.; Tapia, C.V.; Osorio, E.; Velasquez, L.A.; Christodoulides, M. Modified profile of matrix metalloproteinase-2 and -9 production by human Fallopian tube epithelial cells following infection in vitro with Neisseria gonorrhoeae. J. Infect. Dis. 2016. [Google Scholar] [CrossRef]
- Tatti, O.; Gucciardo, E.; Pekkonen, P.; Holopainen, T.; Louhimo, R.; Repo, P.; Maliniemi, P.; Lohi, J.; Rantanen, V.; Hautaniemi, S.; et al. MMP16 Mediates a Proteolytic Switch to Promote Cell-Cell Adhesion, Collagen Alignment, and Lymphatic Invasion in Melanoma. Cancer Res. 2015, 75, 2083–2094. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yu, L.; Sun, C.; Cheng, D.; Yu, S.; Wang, Q.; Yan, Y.; Kang, C.; Jin, S.; et al. miR-146b-5p inhibits glioma migration and invasion by targeting MMP16. Cancer Lett. 2013, 339, 260–269. [Google Scholar] [CrossRef]
- Astarci, E.; Erson-Bensan, A.E.; Banerjee, S. Matrix metalloprotease 16 expression is downregulated by microRNA-146a in spontaneously differentiating Caco-2 cells. Dev. Growth Differ. 2012, 54, 216–226. [Google Scholar] [CrossRef]
- Chen, B.; Huang, Z.; Zhang, Y.; Chen, Y.; Li, Z. MicroRNA-145 Suppresses Osteosarcoma Metastasis via Targeting MMP16. Cell. Physiol. Biochem. 2015, 37, 2183–2193. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-kappaB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef]
- Peteranderl, C.; Sznajder, J.I.; Herold, S.; Lecuona, E. Inflammatory Responses Regulating Alveolar Ion Transport during Pulmonary Infections. Front. Immunol. 2017, 8, 446. [Google Scholar] [CrossRef]
- Nishiguchi, M.; Matsumoto, M.; Takao, T.; Hoshino, M.; Shimonishi, Y.; Tsuji, S.; Begum, N.A.; Takeuchi, O.; Akira, S.; Toyoshima, K.; et al. Mycoplasma fermentans lipoprotein M161Ag-induced cell activation is mediated by Toll-like receptor 2: role of N-terminal hydrophobic portion in its multiple functions. J. Immunol. 2001, 166, 2610–2616. [Google Scholar] [CrossRef]
- Ma, X.; Becker Buscaglia, L.E.; Barker, J.R.; Li, Y. MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 2011, 3, 159–166. [Google Scholar] [CrossRef]
- Ozata, D.M.; Li, X.; Lee, L.; Liu, J.; Warsito, D.; Hajeri, P.; Hultman, I.; Fotouhi, O.; Marklund, S.; Ahrlund-Richter, L.; et al. Loss of miR-514a-3p regulation of PEG3 activates the NF-kappa B pathway in human testicular germ cell tumors. Cell Death Dis. 2017, 8, e2759. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, W.; Cheng, N.; Wang, K.; Li, B.; Jiang, X.; Sun, S. Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology 2012, 56, 1631–1640. [Google Scholar] [CrossRef]
- Wang, P.; Cao, J.; Liu, S.; Pan, H.; Liu, X.; Sui, A.; Wang, L.; Yao, R.; Liu, Z.; Liang, J. Upregulated microRNA-429 inhibits the migration of HCC cells by targeting TRAF6 through the NF-kappaB pathway. Oncol. Rep. 2017, 37, 2883–2890. [Google Scholar] [CrossRef]
- Gu, Y.; Ampofo, E.; Menger, M.D.; Laschke, M.W. miR-191 suppresses angiogenesis by activation of NF-kappaB signaling. FASEB J. 2017. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sanchez-Hidalgo, M.; Cardeno, A.; Rosillo, M.A.; Sanchez-Fidalgo, S.; Utrilla, J.; Martin-Lacave, I.; Alarcon-de-la-Lastra, C. Dietary extra virgin olive oil attenuates kidney injury in pristane-induced SLE model via activation of HO-1/Nrf-2 antioxidant pathway and suppression of JAK/STAT, NF-kappaB and MAPK activation. J. Nutr. Biochem. 2016, 27, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, S.; Abbasirad, N.; Movahed, A.; Khazaei, H.A.; Pishjoo, M.; Rezaei, N. TLR9-based immunotherapy for the treatment of allergic diseases. Immunotherapy 2017, 9, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Vimalanathan, S.; Schoop, R.; Suter, A.; Hudson, J. Prevention of influenza virus induced bacterial superinfection by standardized Echinacea purpurea, via regulation of surface receptor expression in human bronchial epithelial cells. Virus Res. 2017, 233, 51–59. [Google Scholar] [CrossRef]
- Yeh, D.W.; Huang, L.R.; Chen, Y.W.; Huang, C.F.; Chuang, T.H. Interplay between Inflammation and Stemness in Cancer Cells: The Role of Toll-Like Receptor Signaling. J. Immunol. Res. 2016, 2016, 4368101. [Google Scholar] [CrossRef]
- Ghosh, S.; Dass, J.F. Study of pathway cross-talk interactions with NF-kappaB leading to its activation via ubiquitination or phosphorylation: A brief review. Gene 2016, 584, 97–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y. Toll-like receptor-6 (TLR6) deficient mice are protected from myocardial fibrosis induced by high fructose feeding through anti-oxidant and inflammatory signaling pathway. Biochem. Biophys. Res. Commun. 2016, 473, 388–395. [Google Scholar] [CrossRef]
- Monaco, C.; Andreakos, E.; Kiriakidis, S.; Mauri, C.; Bicknell, C.; Foxwell, B.; Cheshire, N.; Paleolog, E.; Feldmann, M. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc. Natl. Acad. Sci. USA 2004, 101, 5634–5639. [Google Scholar] [CrossRef]
- Zhong, J.H.; Li, J.; Liu, C.F.; Liu, N.; Bian, R.X.; Zhao, S.M.; Yan, S.Y.; Zhang, Y.B. Effects of microRNA-146a on the proliferation and apoptosis of human osteoarthritis chondrocytes by targeting TRAF6 through the NF-kappaB signalling pathway. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef]
- Wang, W.M.; Liu, J.C. Effect and molecular mechanism of mir-146a on proliferation of lung cancer cells by targeting and regulating MIF gene. Asian Pac. J. Trop. Med. 2016, 9, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Barker, B.; Bolanos, L.; Liu, X.; Jerez, A.; Makishima, H.; Christie, S.; Chen, X.; Rao, D.S.; Grimes, H.L.; et al. Myeloid malignancies with chromosome 5q deletions acquire a dependency on an intrachromosomal NF-kappaB gene network. Cell Rep. 2014, 8, 1328–1338. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, J.; Zhang, C.Y.; Shen, Y.Q.; Wang, H.M.; Ding, L.; Gu, Y.C.; Lou, J.T.; Zhao, X.T.; Ma, Z.L.; et al. MiR-146a-5p inhibits cell proliferation and cell cycle progression in NSCLC cell lines by targeting CCND1 and CCND2. Oncotarget 2016, 7, 59287–59298. [Google Scholar] [CrossRef]
- Cai, H.H.; Wang, H.Y.; Liu, H.R.; Sheng, Y.J.; Xi, D.G.; Xue, Y.P.; Dai, X.L.; Wang, A.D.; Huang, Q.; Dong, J. Down-regulation of miR-146b-5p promotes malignant transformation of fusion cells after co-culture of macrophages with glioma stem cells in vitro. Zhonghua Yi Xue Za Zhi 2017, 97, 380–386. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Kochumon, S.; Usmani, S.; Sindhu, S.; Ahmad, R. Pam3CSK4 Induces MMP-9 Expression in Human Monocytic THP-1 Cells. Cell. Physiol. Biochem. 2017, 41, 1993–2003. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Z.; Ma, J.; Chen, J.; Zhu, H.; Zhou, X.; Zhu, Q.; Dong, J.; Lan, Q.; Huang, Q. Clinical characteristics and molecular pathology of skull ectopic thyroid cancer. Ann. Transl. Med. 2016, 4, 462. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.T.; Wu, C.; Wu, Z.W.; Li, Y.Y.; Yang, T.Q.; Chen, G.L.; Xie, X.S.; Huang, Y.L.; Du, Z.W.; et al. miR-132 can inhibit glioma cells invasion and migration by target MMP16 in vitro. Onco Targets Ther. 2015, 8, 3211–3218. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Chen, C.; Zhu, H.; Gu, X.; Deng, D.; Tian, X.; Liu, J.; Xiao, Q. MMP16 is a marker of poor prognosis in gastric cancer promoting proliferation and invasion. Oncotarget 2016, 7, 51865–51874. [Google Scholar] [CrossRef] [Green Version]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Han, Y.; Wang, Z.; Zhao, Y.; Fu, Y.; Peng, X. gga-miR-146c Activates TLR6/MyD88/NF-κB Pathway through Targeting MMP16 to Prevent Mycoplasma Gallisepticum (HS Strain) Infection in Chickens. Cells 2019, 8, 501. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8050501
Zhang K, Han Y, Wang Z, Zhao Y, Fu Y, Peng X. gga-miR-146c Activates TLR6/MyD88/NF-κB Pathway through Targeting MMP16 to Prevent Mycoplasma Gallisepticum (HS Strain) Infection in Chickens. Cells. 2019; 8(5):501. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8050501
Chicago/Turabian StyleZhang, Kang, Yun Han, Zaiwei Wang, Yabo Zhao, Yali Fu, and Xiuli Peng. 2019. "gga-miR-146c Activates TLR6/MyD88/NF-κB Pathway through Targeting MMP16 to Prevent Mycoplasma Gallisepticum (HS Strain) Infection in Chickens" Cells 8, no. 5: 501. https://0-doi-org.brum.beds.ac.uk/10.3390/cells8050501