Intrathymic Selection and Defects in the Thymic Epithelial Cell Development
Abstract
:1. Introduction
2. Thymic Epithelium Is Originated from Epithelial Progenitors (TEPCs) whose Nature Is Controversial
3. Cortical Epithelium and Positive Selection
4. mTECs Constitute A Heterogeneous Thymic Cell Population Involved in both Negative Selection and Treg Cell Generation
5. The Condition of EphB-Deficient Thymuses
6. Does the Lack of Eph and/or Ephrins Affect the Thymic Selection?
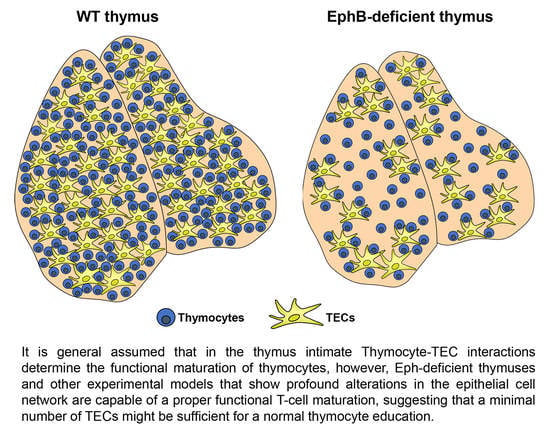
7. How Many TECs Are Necessary for Supporting a Proper T-Cell Maturation?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gallegos, A.M.; Bevan, M.J. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J. Exp. Med. 2004, 200, 1039–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.; Anderson, M.S. Thymic tolerance as a key brake on autoimmunity. Nat. Immunol. 2018, 19, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.J.; Zapata, A.G. Thymus ontogeny and development. In Thymus Transcriptome and Cell Biology; Passos, G.A., Ed.; Springer: Cham, Switzerland, 2019; pp. 19–34. [Google Scholar]
- Passos, G.A.; Genari, A.B.; Assis, A.F.; Monteleone-Cassiano, A.C.; Donadi, E.A.; Oliveira, E.H.; Duarte, M.J.; Machado, M.V.; Tanaka, P.P.; Mascarenhas, R. The thymus as a mirror of the body’s gene expression. In Thymus Transcriptome and Cell Biology; Passos, G.A., Ed.; Springer: Cham, Switzerland, 2019; pp. 215–234. [Google Scholar]
- Singer, A.; Adoro, S.; Park, J.H. Lineage fate and intense debate: Myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat. Rev. Immunol. 2008, 8, 788–801. [Google Scholar] [CrossRef]
- Forster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Derbinski, J.; Schulte, A.; Kyewski, B.; Klein, L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2001, 2, 1032–1039. [Google Scholar] [CrossRef]
- Abramson, J.; Anderson, G. Thymic epithelial cells. Annu. Rev. Immunol. 2017, 35, 85–118. [Google Scholar] [CrossRef]
- Kadouri, N.; Nevo, S.; Goldfarb, Y.; Abramson, J. Thymic epithelial cell heterogeneity: TEC by TEC. Nat. Rev. Immunol. 2020, 20, 239–253. [Google Scholar] [CrossRef]
- Wang, H.X.; Pan, W.; Zheng, L.; Zhong, X.P.; Tan, L.; Liang, Z.; He, J.; Feng, P.; Zhao, Y.; Qiu, Y.R. Thymic epithelial cells contribute to thymopoiesis and T cell development. Front. Immunol. 2019, 10, 3099. [Google Scholar] [CrossRef]
- Takaba, H.; Takayanagi, H. The Mechanisms of T cell selection in the thymus. Trends Immunol. 2017, 38, 805–816. [Google Scholar] [CrossRef]
- Ulyanchenko, S.; O’Neill, K.E.; Medley, T.; Farley, A.M.; Vaidya, H.J.; Cook, A.M.; Blair, N.F.; Blackburn, C.C. Identification of a Bipotent Epithelial Progenitor Population in the Adult Thymus. Cell Rep. 2016, 14, 2819–2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.; Lister, N.L.; Barsanti, M.; Lim, J.M.; Hammett, M.V.; Khong, D.M.; Siatskas, C.; Gray, D.H.; Boyd, R.L.; Chidgey, A.P. Multilineage potential and self-renewal define an epithelial progenitor cell population in the adult thymus. Cell Rep. 2014, 8, 1198–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepletier, A.; Hun, M.L.; Hammett, M.V.; Wong, K.; Naeem, H.; Hedger, M.; Loveland, K.; Chidgey, A.P. Interplay between Follistatin, Activin A, and BMP4 Signaling Regulates Postnatal Thymic Epithelial Progenitor Cell Differentiation during Aging. Cell Rep. 2019, 27, 3887–3901.e4. [Google Scholar] [CrossRef] [Green Version]
- Gill, J.; Malin, M.; Hollander, G.A.; Boyd, R. Generation of a complete thymic microenvironment by MTS24(+) thymic epithelial cells. Nat. Immunol. 2002, 3, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.W.; Jenkinson, W.E.; Anderson, G.; Jenkinson, E.J. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature 2006, 441, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.R.; Farley, A.; Blair, N.F.; Gordon, J.; Sharp, L.; Blackburn, C.C. Identification and characterization of thymic epithelial progenitor cells. Immunity 2002, 16, 803–814. [Google Scholar] [CrossRef] [Green Version]
- Mayer, C.E.; Zuklys, S.; Zhanybekova, S.; Ohigashi, I.; Teh, H.Y.; Sansom, S.N.; Shikama-Dorn, N.; Hafen, K.; Macaulay, I.C.; Deadman, M.E.; et al. Dynamic spatio-temporal contribution of single beta5t+ cortical epithelial precursors to the thymus medulla. Eur. J. Immunol. 2016, 46, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Ohigashi, I.; Zuklys, S.; Sakata, M.; Mayer, C.E.; Hamazaki, Y.; Minato, N.; Hollander, G.A.; Takahama, Y. Adult Thymic Medullary Epithelium Is Maintained and Regenerated by Lineage-Restricted Cells Rather Than Bipotent Progenitors. Cell Rep. 2015, 13, 1432–1443. [Google Scholar] [CrossRef] [Green Version]
- Baik, S.; Jenkinson, E.J.; Lane, P.J.; Anderson, G.; Jenkinson, W.E. Generation of both cortical and Aire(+) medullary thymic epithelial compartments from CD205(+) progenitors. Eur. J. Immunol. 2013, 43, 589–594. [Google Scholar] [CrossRef]
- Ohigashi, I.; Zuklys, S.; Sakata, M.; Mayer, C.E.; Zhanybekova, S.; Murata, S.; Tanaka, K.; Hollander, G.A.; Takahama, Y. Aire-expressing thymic medullary epithelial cells originate from beta5t-expressing progenitor cells. Proc. Natl. Acad. Sci. USA 2013, 110, 9885–9890. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.R.; Rodrigues, P.M.; Meireles, C.; Di Santo, J.P.; Alves, N.L. Thymocyte selection regulates the homeostasis of IL-7-expressing thymic cortical epithelial cells in vivo. J. Immunol. 2013, 191, 1200–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahama, Y.; Ohigashi, I.; Baik, S.; Anderson, G. Generation of diversity in thymic epithelial cells. Nat. Rev. Immunol. 2017, 17, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meireles, C.; Ribeiro, A.R.; Pinto, R.D.; Leitao, C.; Rodrigues, P.M.; Alves, N.L. Thymic crosstalk restrains the pool of cortical thymic epithelial cells with progenitor properties. Eur. J. Immunol. 2017, 47, 958–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornstein, C.; Nevo, S.; Giladi, A.; Kadouri, N.; Pouzolles, M.; Gerbe, F.; David, E.; Machado, A.; Chuprin, A.; Toth, B.; et al. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature 2018, 559, 622–626. [Google Scholar] [CrossRef]
- Dumont-Lagace, M.; Gerbe, H.; Daouda, T.; Laverdure, J.P.; Brochu, S.; Lemieux, S.; Gagnon, E.; Perreault, C. Detection of Quiescent Radioresistant Epithelial Progenitors in the Adult Thymus. Front. Immunol. 2017, 8, 1717. [Google Scholar] [CrossRef]
- Shakib, S.; Desanti, G.E.; Jenkinson, W.E.; Parnell, S.M.; Jenkinson, E.J.; Anderson, G. Checkpoints in the development of thymic cortical epithelial cells. J. Immunol. 2009, 182, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Alves, N.L.; Richard-Le Goff, O.; Huntington, N.D.; Sousa, A.P.; Ribeiro, V.S.; Bordack, A.; Vives, F.L.; Peduto, L.; Chidgey, A.; Cumano, A.; et al. Characterization of the thymic IL-7 niche in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 1512–1517. [Google Scholar] [CrossRef] [Green Version]
- Shitara, S.; Hara, T.; Liang, B.; Wagatsuma, K.; Zuklys, S.; Hollander, G.A.; Nakase, H.; Chiba, T.; Tani-ichi, S.; Ikuta, K. IL-7 produced by thymic epithelial cells plays a major role in the development of thymocytes and TCRgammadelta+ intraepithelial lymphocytes. J. Immunol. 2013, 190, 6173–6179. [Google Scholar] [CrossRef] [Green Version]
- Koch, U.; Fiorini, E.; Benedito, R.; Besseyrias, V.; Schuster-Gossler, K.; Pierres, M.; Manley, N.R.; Duarte, A.; Macdonald, H.R.; Radtke, F. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J. Exp. Med. 2008, 205, 2515–2523. [Google Scholar] [CrossRef]
- Hozumi, K.; Mailhos, C.; Negishi, N.; Hirano, K.; Yahata, T.; Ando, K.; Zuklys, S.; Hollander, G.A.; Shima, D.T.; Habu, S. Delta-like 4 is indispensable in thymic environment specific for T cell development. J. Exp. Med. 2008, 205, 2507–2513. [Google Scholar] [CrossRef] [Green Version]
- Gray, D.H.; Seach, N.; Ueno, T.; Milton, M.K.; Liston, A.; Lew, A.M.; Goodnow, C.C.; Boyd, R.L. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 2006, 108, 3777–3785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.J.; Ahn, S.; Park, C.S.; Holmes, K.L.; Westrup, J.; Chang, C.H.; Kim, M.G. The quantitative assessment of MHC II on thymic epithelium: Implications in cortical thymocyte development. Int. Immunol. 2006, 18, 729–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cejalvo, T.; Munoz, J.J.; Tobajas, E.; Fanlo, L.; Alfaro, D.; Garcia-Ceca, J.; Zapata, A. Ephrin-B-dependent thymic epithelial cell-thymocyte interactions are necessary for correct T cell differentiation and thymus histology organization: Relevance for thymic cortex development. J. Immunol. 2013, 190, 2670–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billiard, F.; Kirshner, J.R.; Tait, M.; Danave, A.; Taheri, S.; Zhang, W.; Waite, J.C.; Olson, K.; Chen, G.; Coetzee, S.; et al. Ongoing Dll4-Notch signaling is required for T-cell homeostasis in the adult thymus. Eur. J. Immunol. 2011, 41, 2207–2216. [Google Scholar] [CrossRef]
- Fiorini, E.; Ferrero, I.; Merck, E.; Favre, S.; Pierres, M.; Luther, S.A.; MacDonald, H.R. Cutting edge: Thymic crosstalk regulates delta-like 4 expression on cortical epithelial cells. J. Immunol. 2008, 181, 8199–8203. [Google Scholar] [CrossRef] [Green Version]
- Tussiwand, R.; Engdahl, C.; Gehre, N.; Bosco, N.; Ceredig, R.; Rolink, A.G. The preTCR-dependent DN3 to DP transition requires Notch signaling, is improved by CXCL12 signaling and is inhibited by IL-7 signaling. Eur. J. Immunol. 2011, 41, 3371–3380. [Google Scholar] [CrossRef]
- Carpenter, A.C.; Bosselut, R. Decision checkpoints in the thymus. Nat. Immunol. 2010, 11, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.T.; Takeuchi, K.; Sun, Z.Y.; Touma, M.; Castro, C.E.; Fahmy, A.; Lang, M.J.; Wagner, G.; Reinherz, E.L. The alphabeta T cell receptor is an anisotropic mechanosensor. J. Biol. Chem. 2009, 284, 31028–31037. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, S.; Ishikawa, E.; Sakuma, M.; Ogata, K.; Sakata-Sogawa, K.; Hiroshima, M.; Wiest, D.L.; Tokunaga, M.; Saito, T. Mechanistic basis of pre-T cell receptor-mediated autonomous signaling critical for thymocyte development. Nat. Immunol. 2006, 7, 67–75. [Google Scholar] [CrossRef]
- Egawa, T.; Tillman, R.E.; Naoe, Y.; Taniuchi, I.; Littman, D.R. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J. Exp. Med. 2007, 204, 1945–1957. [Google Scholar] [CrossRef] [Green Version]
- Murata, S.; Sasaki, K.; Kishimoto, T.; Niwa, S.; Hayashi, H.; Takahama, Y.; Tanaka, K. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science 2007, 316, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Murata, S.; Sasaki, K.; Fujii, H.; Ripen, A.M.; Ishimaru, N.; Koyasu, S.; Tanaka, K.; Takahama, Y. Thymoproteasome shapes immunocompetent repertoire of CD8+ T cells. Immunity 2010, 32, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, K.; Van Laethem, F.; Xing, Y.; Akane, K.; Suzuki, H.; Murata, S.; Tanaka, K.; Jameson, S.C.; Singer, A.; Takahama, Y. TCR affinity for thymoproteasome-dependent positively selecting peptides conditions antigen responsiveness in CD8(+) T cells. Nat. Immunol. 2015, 16, 1069–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, T.; Roth, W.; Wong, P.; Nelson, A.; Farr, A.; Deussing, J.; Villadangos, J.A.; Ploegh, H.; Peters, C.; Rudensky, A.Y. Cathepsin L: Critical role in Ii degradation and CD4 T cell selection in the thymus. Science 1998, 280, 450–453. [Google Scholar] [CrossRef]
- Gommeaux, J.; Gregoire, C.; Nguessan, P.; Richelme, M.; Malissen, M.; Guerder, S.; Malissen, B.; Carrier, A. Thymus-specific serine protease regulates positive selection of a subset of CD4+ thymocytes. Eur. J. Immunol. 2009, 39, 956–964. [Google Scholar] [CrossRef]
- Dhalla, F.; Baran-Gale, J.; Maio, S.; Chappell, L.; Hollander, G.A.; Ponting, C.P. Biologically indeterminate yet ordered promiscuous gene expression in single medullary thymic epithelial cells. EMBO J. 2020, 39, e101828. [Google Scholar] [CrossRef]
- Hamazaki, Y.; Fujita, H.; Kobayashi, T.; Choi, Y.; Scott, H.S.; Matsumoto, M.; Minato, N. Medullary thymic epithelial cells expressing Aire represent a unique lineage derived from cells expressing claudin. Nat. Immunol. 2007, 8, 304–311. [Google Scholar] [CrossRef]
- Miller, C.N.; Proekt, I.; von Moltke, J.; Wells, K.L.; Rajpurkar, A.R.; Wang, H.; Rattay, K.; Khan, I.S.; Metzger, T.C.; Pollack, J.L.; et al. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature 2018, 559, 627–631. [Google Scholar] [CrossRef]
- White, A.J.; Withers, D.R.; Parnell, S.M.; Scott, H.S.; Finke, D.; Lane, P.J.; Jenkinson, E.J.; Anderson, G. Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct cellular input. Eur. J. Immunol. 2008, 38, 942–947. [Google Scholar] [CrossRef]
- Gray, D.; Abramson, J.; Benoist, C.; Mathis, D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J. Exp. Med. 2007, 204, 2521–2528. [Google Scholar] [CrossRef] [Green Version]
- Michel, C.; Miller, C.N.; Kuchler, R.; Brors, B.; Anderson, M.S.; Kyewski, B.; Pinto, S. Revisiting the Road Map of Medullary Thymic Epithelial Cell Differentiation. J. Immunol. 2017, 199, 3488–3503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzger, T.C.; Khan, I.S.; Gardner, J.M.; Mouchess, M.L.; Johannes, K.P.; Krawisz, A.K.; Skrzypczynska, K.M.; Anderson, M.S. Lineage tracing and cell ablation identify a post-Aire-expressing thymic epithelial cell population. Cell Rep. 2013, 5, 166–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, Y.; Nishijima, H.; Matsumoto, M.; Morimoto, J.; Hirota, F.; Takahashi, S.; Luche, H.; Fehling, H.J.; Mouri, Y.; Matsumoto, M. Temporal lineage tracing of Aire-expressing cells reveals a requirement for Aire in their maturation program. J. Immunol. 2014, 192, 2585–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onder, L.; Nindl, V.; Scandella, E.; Chai, Q.; Cheng, H.W.; Caviezel-Firner, S.; Novkovic, M.; Bomze, D.; Maier, R.; Mair, F.; et al. Alternative NF-kappaB signaling regulates mTEC differentiation from podoplanin-expressing precursors in the cortico-medullary junction. Eur. J. Immunol. 2015, 45, 2218–2231. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Laan, M.; Bichele, R.; Kisand, K.; Scott, H.S.; Peterson, P. Post-Aire maturation of thymic medullary epithelial cells involves selective expression of keratinocyte-specific autoantigens. Front. Immunol. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miragaia, R.J.; Zhang, X.; Gomes, T.; Svensson, V.; Ilicic, T.; Henriksson, J.; Kar, G.; Lonnberg, T. Single-cell RNA-sequencing resolves self-antigen expression during mTEC development. Sci. Rep. 2018, 8, 685. [Google Scholar] [CrossRef]
- Rossi, S.W.; Kim, M.Y.; Leibbrandt, A.; Parnell, S.M.; Jenkinson, W.E.; Glanville, S.H.; McConnell, F.M.; Scott, H.S.; Penninger, J.M.; Jenkinson, E.J.; et al. RANK signals from CD4(+)3(−) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J. Exp. Med. 2007, 204, 1267–1272. [Google Scholar] [CrossRef]
- Akiyama, T.; Shimo, Y.; Yanai, H.; Qin, J.; Ohshima, D.; Maruyama, Y.; Asaumi, Y.; Kitazawa, J.; Takayanagi, H.; Penninger, J.M.; et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 2008, 29, 423–437. [Google Scholar] [CrossRef] [Green Version]
- Hikosaka, Y.; Nitta, T.; Ohigashi, I.; Yano, K.; Ishimaru, N.; Hayashi, Y.; Matsumoto, M.; Matsuo, K.; Penninger, J.M.; Takayanagi, H.; et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 2008, 29, 438–450. [Google Scholar] [CrossRef] [Green Version]
- Irla, M.; Hugues, S.; Gill, J.; Nitta, T.; Hikosaka, Y.; Williams, I.R.; Hubert, F.X.; Scott, H.S.; Takahama, Y.; Hollander, G.A.; et al. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity 2008, 29, 451–463. [Google Scholar] [CrossRef]
- Finnish-German, A.C. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat. Genet. 1997, 17, 399–403. [Google Scholar]
- Nagamine, K.; Peterson, P.; Scott, H.S.; Kudoh, J.; Minoshima, S.; Heino, M.; Krohn, K.J.; Lalioti, M.D.; Mullis, P.E.; Antonarakis, S.E.; et al. Positional cloning of the APECED gene. Nat. Genet. 1997, 17, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Perheentupa, J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J. Clin. Endocrinol. Metab. 2006, 91, 2843–2850. [Google Scholar] [CrossRef] [Green Version]
- Kisand, K.; Boe Wolff, A.S.; Podkrajsek, K.T.; Tserel, L.; Link, M.; Kisand, K.V.; Ersvaer, E.; Perheentupa, J.; Erichsen, M.M.; Bratanic, N.; et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J. Exp. Med. 2010, 207, 299–308. [Google Scholar] [CrossRef]
- Puel, A.; Doffinger, R.; Natividad, A.; Chrabieh, M.; Barcenas-Morales, G.; Picard, C.; Cobat, A.; Ouachee-Chardin, M.; Toulon, A.; Bustamante, J.; et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 2010, 207, 291–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meloni, A.; Furcas, M.; Cetani, F.; Marcocci, C.; Falorni, A.; Perniola, R.; Pura, M.; Boe Wolff, A.S.; Husebye, E.S.; Lilic, D.; et al. Autoantibodies against type I interferons as an additional diagnostic criterion for autoimmune polyendocrine syndrome type I. J. Clin. Endocrinol. Metab. 2008, 93, 4389–4397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liston, A.; Lesage, S.; Wilson, J.; Peltonen, L.; Goodnow, C.C. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 2003, 4, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.S.; Venanzi, E.S.; Chen, Z.; Berzins, S.P.; Benoist, C.; Mathis, D. The cellular mechanism of Aire control of T cell tolerance. Immunity 2005, 23, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, R.T.; DeVoss, J.J.; Moon, J.J.; Sidney, J.; Sette, A.; Jenkins, M.K.; Anderson, M.S. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proc. Natl. Acad. Sci. USA 2012, 109, 7847–7852. [Google Scholar] [CrossRef] [Green Version]
- Malchow, S.; Leventhal, D.S.; Nishi, S.; Fischer, B.I.; Shen, L.; Paner, G.P.; Amit, A.S.; Kang, C.; Geddes, J.E.; Allison, J.P.; et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science 2013, 339, 1219–1224. [Google Scholar] [CrossRef] [Green Version]
- Perry, J.S.A.; Lio, C.J.; Kau, A.L.; Nutsch, K.; Yang, Z.; Gordon, J.I.; Murphy, K.M.; Hsieh, C.S. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 2014, 41, 414–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Fujikado, N.; Kolodin, D.; Benoist, C.; Mathis, D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 2015, 348, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyewski, B.; Derbinski, J. Self-representation in the thymus: An extended view. Nat. Rev. Immunol. 2004, 4, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Passos, G.A.; Speck-Hernandez, C.A.; Assis, A.F.; Mendes-da-Cruz, D.A. Update on Aire and thymic negative selection. Immunology 2018, 153, 10–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansom, S.N.; Shikama-Dorn, N.; Zhanybekova, S.; Nusspaumer, G.; Macaulay, I.C.; Deadman, M.E.; Heger, A.; Ponting, C.P.; Hollander, G.A. Population and single-cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res. 2014, 24, 1918–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, M.; Zemmour, D.; Mathis, D.; Benoist, C. Aire controls gene expression in the thymic epithelium with ordered stochasticity. Nat. Immunol. 2015, 16, 942–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennecke, P.; Reyes, A.; Pinto, S.; Rattay, K.; Nguyen, M.; Kuchler, R.; Huber, W.; Kyewski, B.; Steinmetz, L.M. Single-cell transcriptome analysis reveals coordinated ectopic gene-expression patterns in medullary thymic epithelial cells. Nat. Immunol 2015, 16, 933–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.S.; Venanzi, E.S.; Klein, L.; Chen, Z.; Berzins, S.P.; Turley, S.J.; von Boehmer, H.; Bronson, R.; Dierich, A.; Benoist, C.; et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 2002, 298, 1395–1401. [Google Scholar] [CrossRef] [Green Version]
- Takaba, H.; Morishita, Y.; Tomofuji, Y.; Danks, L.; Nitta, T.; Komatsu, N.; Kodama, T.; Takayanagi, H. Fezf2 Orchestrates a Thymic Program of Self-Antigen Expression for Immune Tolerance. Cell 2015, 163, 975–987. [Google Scholar] [CrossRef] [Green Version]
- Cosway, E.J.; Lucas, B.; James, K.D.; Parnell, S.M.; Carvalho-Gaspar, M.; White, A.J.; Tumanov, A.V.; Jenkinson, W.E.; Anderson, G. Redefining thymus medulla specialization for central tolerance. J. Exp. Med. 2017, 214, 3183–3195. [Google Scholar] [CrossRef]
- Bonasio, R.; Scimone, M.L.; Schaerli, P.; Grabie, N.; Lichtman, A.H.; von Andrian, U.H. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat. Immunol. 2006, 7, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Hadeiba, H.; Butcher, E.C. Thymus-homing dendritic cells in central tolerance. Eur. J. Immunol. 2013, 43, 1425–1459. [Google Scholar] [CrossRef] [Green Version]
- Leventhal, D.S.; Gilmore, D.C.; Berger, J.M.; Nishi, S.; Lee, V.; Malchow, S.; Kline, D.E.; Kline, J.; Vander Griend, D.J.; Huang, H.; et al. Dendritic Cells Coordinate the Development and Homeostasis of Organ-Specific Regulatory T Cells. Immunity 2016, 44, 847–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbongue, J.; Nicholas, D.; Firek, A.; Langridge, W. The role of dendritic cells in tissue-specific autoimmunity. J. Immunol. Res. 2014, 2014, 857143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proietto, A.I.; van Dommelen, S.; Zhou, P.; Rizzitelli, A.; D’Amico, A.; Steptoe, R.J.; Naik, S.H.; Lahoud, M.H.; Liu, Y.; Zheng, P.; et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl. Acad. Sci. USA 2008, 105, 19869–19874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubert, F.X.; Kinkel, S.A.; Davey, G.M.; Phipson, B.; Mueller, S.N.; Liston, A.; Proietto, A.I.; Cannon, P.Z.; Forehan, S.; Smyth, G.K.; et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood 2011, 118, 2462–2472. [Google Scholar] [CrossRef] [PubMed]
- Mouri, Y.; Ueda, Y.; Yamano, T.; Matsumoto, M.; Tsuneyama, K.; Kinashi, T.; Matsumoto, M. Mode of Tolerance Induction and Requirement for Aire Are Governed by the Cell Types That Express Self-Antigen and Those That Present Antigen. J. Immunol. 2017, 199, 3959–3971. [Google Scholar] [CrossRef]
- Yano, M.; Kuroda, N.; Han, H.; Meguro-Horike, M.; Nishikawa, Y.; Kiyonari, H.; Maemura, K.; Yanagawa, Y.; Obata, K.; Takahashi, S.; et al. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J. Exp. Med. 2008, 205, 2827–2838. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Hirota, F.; Yano, M.; Kitajima, H.; Miyazaki, J.; Kawamoto, H.; Mouri, Y.; Matsumoto, M. Biphasic Aire expression in early embryos and in medullary thymic epithelial cells before end-stage terminal differentiation. J. Exp. Med. 2010, 207, 963–971. [Google Scholar] [CrossRef] [Green Version]
- Odaka, C.; Hauri-Hohl, M.; Takizawa, K.; Nishikawa, Y.; Yano, M.; Matsumoto, M.; Boyd, R.; Hollander, G.A. TGF-beta type II receptor expression in thymic epithelial cells inhibits the development of Hassall’s corpuscles in mice. Int. Immunol. 2013, 25, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, N.; Wang, Y.H.; Lee, H.K.; Ito, T.; Wang, Y.H.; Cao, W.; Liu, Y.J. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 2005, 436, 1181–1185. [Google Scholar] [CrossRef]
- Banerjee, A.; McKinley, E.T.; von Moltke, J.; Coffey, R.J.; Lau, K.S. Interpreting heterogeneity in intestinal tuft cell structure and function. J. Clin. Investig. 2018, 128, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Gerbe, F.; Legraverend, C.; Jay, P. The intestinal epithelium tuft cells: Specification and function. Cell. Mol. Life Sci. 2012, 69, 2907–2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panneck, A.R.; Rafiq, A.; Schutz, B.; Soultanova, A.; Deckmann, K.; Chubanov, V.; Gudermann, T.; Weihe, E.; Krasteva-Christ, G.; Grau, V.; et al. Cholinergic epithelial cell with chemosensory traits in murine thymic medulla. Cell Tissue Res. 2014, 358, 737–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soultanova, A.; Voigt, A.; Chubanov, V.; Gudermann, T.; Meyerhof, W.; Boehm, U.; Kummer, W. Cholinergic chemosensory cells of the thymic medulla express the bitter receptor Tas2r131. Int. Immunopharmacol. 2015, 29, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.J.; Garcia-Ceca, J.; Montero-Herradon, S.; Sanchez Del Collado, B.; Alfaro, D.; Zapata, A. Can a Proper T-Cell Development Occur in an Altered Thymic Epithelium? Lessons from EphB-Deficient Thymi. Front. Endocrinol. 2018, 9, 135. [Google Scholar] [CrossRef]
- Osada, M.; Ito, E.; Fermin, H.A.; Vazquez-Cintron, E.; Venkatesh, T.; Friedel, R.H.; Pezzano, M. The Wnt signaling antagonist Kremen1 is required for development of thymic architecture. Clin. Dev. Immunol. 2006, 13, 299–319. [Google Scholar] [CrossRef]
- Revest, J.M.; Suniara, R.K.; Kerr, K.; Owen, J.J.; Dickson, C. Development of the thymus requires signaling through the fibroblast growth factor receptor R2-IIIb. J. Immunol. 2001, 167, 1954–1961. [Google Scholar] [CrossRef] [Green Version]
- Lomada, D.; Jain, M.; Bolner, M.; Reeh, K.A.; Kang, R.; Reddy, M.C.; DiGiovanni, J.; Richie, E.R. Stat3 Signaling Promotes Survival and Maintenance of Medullary Thymic Epithelial Cells. PLoS Genet. 2016, 12, e1005777. [Google Scholar] [CrossRef] [Green Version]
- Munoz, J.J.; Alonso, C.L.; Sacedon, R.; Crompton, T.; Vicente, A.; Jimenez, E.; Varas, A.; Zapata, A.G. Expression and function of the Eph A receptors and their ligands ephrins A in the rat thymus. J. Immunol. 2002, 169, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Ceca, J.; Alfaro, D.; Montero-Herradon, S.; Tobajas, E.; Munoz, J.J.; Zapata, A.G. Eph/Ephrins-Mediated Thymocyte-Thymic Epithelial Cell Interactions Control Numerous Processes of Thymus Biology. Front. Immunol. 2015, 6, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Himanen, J.P.; Saha, N.; Nikolov, D.B. Cell-cell signaling via Eph receptors and ephrins. Curr. Opin. Cell. Biol. 2007, 19, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Pasquale, E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005, 6, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Knoll, B.; Drescher, U. Ephrin-As as receptors in topographic projections. Trends Neurosci. 2002, 25, 145–149. [Google Scholar] [CrossRef]
- Alfaro, D.; Munoz, J.J.; Garcia-Ceca, J.; Cejalvo, T.; Jimenez, E.; Zapata, A. Alterations in the thymocyte phenotype of EphB-deficient mice largely affect the double negative cell compartment. Immunology 2008, 125, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Montero-Herradon, S.; Garcia-Ceca, J.; Sanchez Del Collado, B.; Alfaro, D.; Zapata, A.G. Eph/ephrin-B-mediated cell-to-cell interactions govern MTS20(+) thymic epithelial cell development. Histochem. Cell Biol. 2016, 146, 167–182. [Google Scholar] [CrossRef]
- Garcia-Ceca, J.; Jimenez, E.; Alfaro, D.; Cejalvo, T.; Chumley, M.J.; Henkemeyer, M.; Munoz, J.J.; Zapata, A.G. On the role of Eph signalling in thymus histogenesis; EphB2/B3 and the organizing of the thymic epithelial network. Int. J. Dev. Biol. 2009, 53, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Montero-Herradon, S.; Garcia-Ceca, J.; Zapata, A.G. EphB receptors, mainly EphB3, contribute to the proper development of cortical thymic epithelial cells. Organogenesis 2017, 13, 192–211. [Google Scholar] [CrossRef]
- Stimamiglio, M.A.; Jimenez, E.; Silva-Barbosa, S.D.; Alfaro, D.; Garcia-Ceca, J.J.; Munoz, J.J.; Cejalvo, T.; Savino, W.; Zapata, A. EphB2-mediated interactions are essential for proper migration of T cell progenitors during fetal thymus colonization. J. Leukoc. Biol. 2010, 88, 483–494. [Google Scholar] [CrossRef]
- Alfaro, D.; Garcia-Ceca, J.; Farias-de-Oliveira, D.A.; Terra-Granado, E.; Montero-Herradon, S.; Cotta-de-Almeida, V.; Savino, W.; Zapata, A. EphB2 and EphB3 play an important role in the lymphoid seeding of murine adult thymus. J. Leukoc. Biol. 2015, 98, 883–896. [Google Scholar] [CrossRef]
- Luo, H.; Wu, Z.; Qi, S.; Jin, W.; Han, B.; Wu, J. Ephrinb1 and Ephrinb2 are associated with interleukin-7 receptor alpha and retard its internalization from the cell surface. J. Biol. Chem. 2011, 286, 44976–44987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, W.E.; Jenkinson, E.J.; Anderson, G. Differential requirement for mesenchyme in the proliferation and maturation of thymic epithelial progenitors. J. Exp. Med. 2003, 198, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Ceca, J.; Alfaro, D.; Montero-Herradon, S.; Zapata, A.G. Eph/ephrinB signalling is involved in the survival of thymic epithelial cells. Immunol. Cell Biol. 2013, 91, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Rodewald, H.R.; Paul, S.; Haller, C.; Bluethmann, H.; Blum, C. Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature 2001, 414, 763–768. [Google Scholar] [CrossRef]
- Montero-Herradon, S.; Garcia-Ceca, J.; Zapata, A.G. Altered Maturation of Medullary TEC in EphB-Deficient Thymi Is Recovered by RANK Signaling Stimulation. Front. Immunol. 2018, 9, 1020. [Google Scholar] [CrossRef]
- Garcia-Ceca, J.; Montero-Herradon, S.; Alfaro, D.; Zapata, A.G. Increased epithelial-free areas in thymuses with altered EphB-mediated thymocyte-thymic epithelial cell interactions. Histochem. Cell Biol. 2017, 148, 381–394. [Google Scholar] [CrossRef]
- Lucas, B.; McCarthy, N.I.; Baik, S.; Cosway, E.; James, K.D.; Parnell, S.M.; White, A.J.; Jenkinson, W.E.; Anderson, G. Control of the thymic medulla and its influence on alphabetaT-cell development. Immunol. Rev. 2016, 271, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Mouri, Y.; Yano, M.; Shinzawa, M.; Shimo, Y.; Hirota, F.; Nishikawa, Y.; Nii, T.; Kiyonari, H.; Abe, T.; Uehara, H.; et al. Lymphotoxin signal promotes thymic organogenesis by eliciting RANK expression in the embryonic thymic stroma. J. Immunol. 2011, 186, 5047–5057. [Google Scholar] [CrossRef]
- McCarthy, N.I.; Cowan, J.E.; Nakamura, K.; Bacon, A.; Baik, S.; White, A.J.; Parnell, S.M.; Jenkinson, E.J.; Jenkinson, W.E.; Anderson, G. Osteoprotegerin-Mediated Homeostasis of Rank+ Thymic Epithelial Cells Does Not Limit Foxp3+ Regulatory T Cell Development. J. Immunol. 2015, 195, 2675–2682. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Luo, H.; Wu, J. Effect of reduced EPHB4 expression in thymic epithelial cells on thymocyte development and peripheral T cell function. Mol. Immunol. 2014, 58, 1–9. [Google Scholar] [CrossRef]
- Shimoyama, M.; Matsuoka, H.; Nagata, A.; Iwata, N.; Tamekane, A.; Okamura, A.; Gomyo, H.; Ito, M.; Jishage, K.; Kamada, N.; et al. Developmental expression of EphB6 in the thymus: Lessons from EphB6 knockout mice. Biochem. Biophys. Res. Commun. 2002, 298, 87–94. [Google Scholar] [CrossRef]
- Kawano, H.; Katayama, Y.; Minagawa, K.; Shimoyama, M.; Henkemeyer, M.; Matsui, T. A novel feedback mechanism by Ephrin-B1/B2 in T-cell activation involves a concentration-dependent switch from costimulation to inhibition. Eur. J. Immunol. 2012, 42, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Qi, S.; Luo, H. The effect of conditional EFNB1 deletion in the T cell compartment on T cell development and function. BMC Immunol. 2011, 12, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, W.; Qi, S.; Luo, H. T cell-specific deletion of EFNB2 minimally affects T cell development and function. Mol. Immunol. 2012, 52, 141–147. [Google Scholar] [CrossRef]
- Luo, H.; Broux, B.; Wang, X.; Hu, Y.; Ghannam, S.; Jin, W.; Larochelle, C.; Prat, A.; Wu, J. EphrinB1 and EphrinB2 regulate T cell chemotaxis and migration in experimental autoimmune encephalomyelitis and multiple sclerosis. Neurobiol. Dis. 2016, 91, 292–306. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Wu, Y.; Jin, W.; Cheng, B.; Fang, X.; Martel-Pelletier, J.; Kapoor, M.; Peng, J.; Qi, S.; et al. Role of EFNB1 and EFNB2 in Mouse Collagen-Induced Arthritis and Human Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 1778–1788. [Google Scholar] [CrossRef] [Green Version]
- Munoz, J.J.; Alfaro, D.; Garcia-Ceca, J.; Alonso, C.L.; Jimenez, E.; Zapata, A. Thymic alterations in EphA4-deficient mice. J. Immunol. 2006, 177, 804–813. [Google Scholar] [CrossRef] [Green Version]
- Bruserud, O.; Oftedal, B.E.; Wolff, A.B.; Husebye, E.S. AIRE-mutations and autoimmune disease. Curr. Opin. Immunol. 2016, 43, 8–15. [Google Scholar] [CrossRef]
- Gimenez-Barcons, M.; Casteras, A.; Armengol Mdel, P.; Porta, E.; Correa, P.A.; Marin, A.; Pujol-Borrell, R.; Colobran, R. Autoimmune predisposition in Down syndrome may result from a partial central tolerance failure due to insufficient intrathymic expression of AIRE and peripheral antigens. J. Immunol. 2014, 193, 3872–3879. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Ohigashi, I.; Nitta, T.; Sakata, M.; Tanaka, K.; Murata, S.; Kanagawa, O.; Takahama, Y. Thymic nurse cells provide microenvironment for secondary T cell receptor alpha rearrangement in cortical thymocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 20572–20577. [Google Scholar] [CrossRef] [Green Version]
- Alfaro, D.; Munoz, J.J.; Garcia-Ceca, J.; Cejalvo, T.; Jimenez, E.; Zapata, A.G. The Eph/ephrinB signal balance determines the pattern of T-cell maturation in the thymus. Immunol. Cell Biol. 2011, 89, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Liston, A.; Gray, D.H.; Lesage, S.; Fletcher, A.L.; Wilson, J.; Webster, K.E.; Scott, H.S.; Boyd, R.L.; Peltonen, L.; Goodnow, C.C. Gene dosage—limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J. Exp. Med. 2004, 200, 1015–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, M.A.; Giang, K.; Zumer, K.; Jiang, H.; Oven, I.; Rinn, J.L.; Devoss, J.J.; Johannes, K.P.; Lu, W.; Gardner, J.; et al. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. J. Clin. Investig. 2008, 118, 1712–1726. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Kyewski, B. Self-antigen presentation by thymic stromal cells: A subtle division of labor. Curr. Opin. Immunol. 2000, 12, 179–186. [Google Scholar] [CrossRef]
- Lopes, N.; Serge, A.; Ferrier, P.; Irla, M. Thymic Crosstalk Coordinates Medulla Organization and T-Cell Tolerance Induction. Front. Immunol. 2015, 6, 365. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, H.; Matsumoto, T. Mechanisms of Tolerance Induction by Dendritic Cells In Vivo. Front. Immunol. 2018, 9, 350. [Google Scholar] [CrossRef]
- Garcia-Ceca, J.; Montero-Herradon, S.; Zapata, A.G. Thymus aging in mice deficient in either EphB2 or EphB3, two master regulators of thymic epithelium development. Dev. Dyn. 2020. [Google Scholar] [CrossRef]
- Prockop, S.E.; Petrie, H.T. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J. Immunol. 2004, 173, 1604–1611. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, W.E.; Rossi, S.W.; Parnell, S.M.; Jenkinson, E.J.; Anderson, G. PDGFRalpha-expressing mesenchyme regulates thymus growth and the availability of intrathymic niches. Blood 2007, 109, 954–960. [Google Scholar] [CrossRef]




| RTOC (×106 TSC) | After Graftment (×106 cells) | % CD4+ (CD4+CD8−) | % DP (CD4+CD8+) | % DN (CD4−CD8−) | % CD8+ (CD4−CD8+) |
|---|---|---|---|---|---|
| 1 | 5.05 ± 2.71 | 7.00 ± 2.27 | 87.95 ± 2.26 | 2.11 ± 0.10 | 2.94 ± 0.21 |
| 0.5 | 3.54 ± 2.41 | 8.94 ± 0.68 | 87.56 ± 0.59 | 1.00 ± 0.43 | 2.51 ± 0.85 |
| 0.25 | 1.03 ± 0.70 | 10.24 ± 1.50 | 84.24 ± 2.17 | 2.08 ± 0.26 | 3.45 ± 0.41 |
| 0.1 | 0.34 ± 0.18 * | 8.96 ± 3.59 | 86.24 ± 5.46 | 3.11 ± 2.23 | 1.70 ± 1.01 |
| 0.085 | 0.26 ± 0.23 * | 16.44 ± 3.80 * | 76.49 ± 4.11 * | 2.36 ± 0.78 | 4.72 ± 1.81 |
| RTOC(×106 TSC) | % of Total TCRαβhi | % of Total TCRαβhiCD4+ | % of Total TCRαβhiCD8+ | % of Total TCRαβhiCD4+CD8+ |
|---|---|---|---|---|
| 1 | 9.20 ± 3.09 | 5.51 ± 1.96 | 1.36 ± 0.37 | 2.11 ± 0.72 |
| 0.5 | 11.03 ± 1.01 | 7.05 ± 0.46 | 1.57 ± 0.07 | 2.30 ± 0.50 |
| 0.25 | 13.17 ± 0.93 | 8.38 ± 0.97 | 1.93 ± 0.54 | 2.38 ± 0.47 |
| 0.1 | 9.49 ± 3.83 | 5.73 ± 2.42 | 1.15 ± 0.66 | 1.94 ± 0.15 |
| 0.085 | 20.76 ± 3.53 ** | 14.00 ± 3.21 ** | 3.11 ± 0.67 * | 3.31 ± 0.44 |
| RTOC (×106 TSC) | % of CD69+ within TCRαβhi Cells | % of CD69+ within CD4+ Gated in TCRαβhi Cells | % of Total TCRαβhiCD69+ | % of Total TCRαβhiCD69+CD4+ |
|---|---|---|---|---|
| 1 | 55.06 ± 4.98 | 59.19 ± 5.23 | 4.76 ± 1.51 | 3.13 ± 1.09 |
| 0.5 | 49.84 ± 8.53 | 53.23 ± 9.16 | 5.42 ± 0.02 | 3.62 ± 0.45 |
| 0.25 | 46.62 ± 4.69 | 50.22 ± 1.46 | 6.99 ± 2.23 | 4.85 ± 1.65 |
| 0.1 | 45.45 ± 8.40 | 47.05 ± 12.12 | 4.51 ± 1.11 | 2.92 ± 1.06 |
| 0.085 | 39.02 ± 3.75 * | 37.72 ± 3.62 * | 8.49 ± 2.73 | 5.48 ± 2.18 |
| RTOC (×106 TSC) | % of Foxp3+ within TCRαβhi Cells | % of Foxp3+ within CD4+ Gated in TCRαβhi Cells | % of Total TCRαβhiFoxp3+ | % of Total TCRαβhiFoxp3+CD4+ |
|---|---|---|---|---|
| 1 | 3.24 ± 0.12 | 3.63 ± 0.39 | 0.25 ± 0.07 | 0.18 ± 0.05 |
| 0.5 | 3.73 ± 1.29 | 4.47 ± 1.68 | 0.36 ± 0.15 | 0.28 ± 0.10 |
| 0.25 | 2.64 ± 0.58 | 3.04 ± 0.71 | 0.30 ± 0.04 | 0.25 ± 0.03 |
| 0.1 | 3.24 ± 0.23 | 3.64 ± 0.13 | 0.23 ± 0.01 | 0.15 ± 0.01 |
| 0.085 | 3.46 ± 1.52 | 4.06 ± 2.25 | 0.57 ± 0.15 * | 0.44 ± 0.12 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Ceca, J.; Montero-Herradón, S.; Zapata, A.G. Intrathymic Selection and Defects in the Thymic Epithelial Cell Development. Cells 2020, 9, 2226. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9102226
García-Ceca J, Montero-Herradón S, Zapata AG. Intrathymic Selection and Defects in the Thymic Epithelial Cell Development. Cells. 2020; 9(10):2226. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9102226
Chicago/Turabian StyleGarcía-Ceca, Javier, Sara Montero-Herradón, and Agustín G. Zapata. 2020. "Intrathymic Selection and Defects in the Thymic Epithelial Cell Development" Cells 9, no. 10: 2226. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9102226