Crosstalk Pathway between Trehalose Metabolism and Cytokinin Degradation for the Determination of the Number of Berries per Bunch in Grapes
Abstract
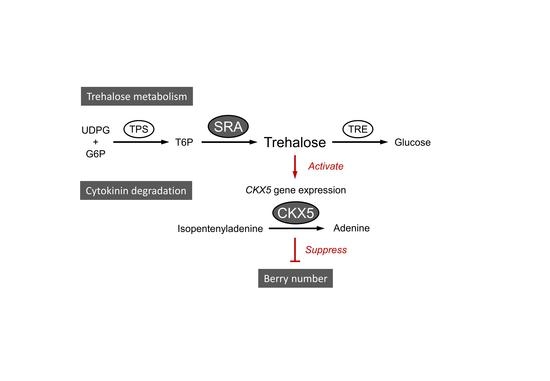
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Number of Berries per Bunch
2.3. Trehalose Treatment of Grapevine Cultured Cells
2.4. Trehalose Treatment of Juvenile Grape Inflorescences
2.5. NAA and Kinetin Treatment of Grapevine Cultured Cells
2.6. RNA Isolation
2.7. Real-Time RT-PCR
2.8. Simple Linear Regression Analysis
2.9. Overexpression of VvCKX5 in Arabidopsis Plants
2.10. Phenotypic Analysis of VvCKX5-Overexpressing Arabidopsis Plants
2.11. Trehalose Treatment of Buds of Field-Grown Grapevines
2.12. Bunch and Berry Characteristics
2.13. Statistical Analysis
3. Results
3.1. Number of Berries per Bunch Differs among Grape Cultivars
3.2. VvSRA Overexpression Increases Cytokinin Oxidase/Hydrogenase Expression in Arabidopsis Plants
3.3. VvCKX5 Gene Expression Is Negatively Correlated with Number of Berries per Bunch
3.4. Trehalose Upregulates VvCKX5 Gene Expression in Grapevine Cultured Cells and Juvenile Grape Inflorescences
3.5. NAA Upregulates VvCKX5 Gene Expression in Grapevine Cultured Cells
3.6. VvCKX5 Overexpression Has a Notable Effect on Flower Number in Arabidopsis Plants
3.7. Trehalose Injection into Buds Decreases the Number of Berries per Bunch
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPGRI; UPOV; OIV. Descriptors for Grapevine (Vitis spp.); International Union for the Protection of New Varieties of Plants: Geneva, Switzerland; Office International de la Vigne et du Vin: Paris, France; International Plant Genetic Resources Institute: Rome, Italy, 1997. [Google Scholar]
- Gao, X.T.; Wu, M.H.; Sun, D.; Li, H.Q.; Chen, W.K.; Yang, H.; Liu, F.Q.; Wang, Q.C.; Wang, Y.Y.; Wang, J.; et al. Effects of gibberellic acid (GA3) application before anthesis on rachis elongation and berry quality and aroma and flavour compounds in Vitis vinifera L. ‘Cabernet Franc’ and ‘Cabernet Sauvignon’ grapes. J. Sci. Food Agric. 2020, 100, 3729–3740. [Google Scholar] [CrossRef] [PubMed]
- Miele, A.; Weaver, R.J.; Johnson, J. Effect of potassium gibberellate on fruit-set and development of Thompson Seedless and Zinfandel grapes. Am. J. Enol. Vitic. 1978, 29, 79–82. [Google Scholar]
- Nagao, A.; Sato, M. Effects of gibberellic acid spraying on peduncle elongation of Riesling grape. J. ASEV Jpn. 1999, 10, 12–19. [Google Scholar]
- Tello, J.; Forneck, A. A double-sigmoid model for grapevine bunch compactness development. OENO ONE 2018, 52, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Guilpart, N.; Metay, A.; Gary, C. Grapevine bud fertility and number of berries per bunch are determined by water and nitrogen stress around flowering in the previous year. Eur. J. Agron. 2014, 54, 9–20. [Google Scholar] [CrossRef]
- Li-Mallet, A.; Rabot, A.; Geny, L. Factors controlling inflorescence primordia formation of grapevine: Their role in latent bud fruitfulness? A review. Botany 2016, 94, 147–163. [Google Scholar] [CrossRef]
- Tello, J.; Aguirrezábal, R.; Hernaiz, S.; Larreina, B.; Montemayor, M.I.; Vaquero, E.; Ibáñez, J. Multicultivar and multivariate study of the natural variation for grapevine bunch compactness. Aust. J. Grape Wine Res. 2015, 21, 277–289. [Google Scholar] [CrossRef]
- Tello, J.; Torres-Pérez, R.; Grimplet, J.; Ibáñez, J. Association analysis of grapevine bunch traits using a comprehensive approach. Theor. Appl. Genet. 2016, 129, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Gallavotti, A.; Long, J.A.; Stanfield, S.; Yang, X.; Jackson, D.; Vollbrecht, E.; Schmidt, R.J. The control of axillary meristem fate in the maize ramosa pathway. Development 2010, 137, 2849–2856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh-Nagasawa, N.; Nagasawa, N.; Malcomber, S.; Sakai, H.; Jackson, D. A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 2006, 441, 227–230. [Google Scholar] [CrossRef]
- Vollbrecht, E.; Springer, P.S.; Goh, L.; Buckler, E.S., IV; Martienssen, R. Architecture of floral branch systems in maize and related grasses. Nature 2005, 436, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Ishiai, S.; Nakajima, Y.; Enoki, S.; Suzuki, S. Grape sister of Ramosa3 is a negative regulator of pedicel development of grape inflorescence. Plant Cell Tissue Organ. Cult. 2016, 124, 217–225. [Google Scholar] [CrossRef]
- Crowe, J.H.; Hoekstra, F.A.; Crowe, L.M. Anhydrobiosis. Annu. Rev. Physiol. 1992, 54, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Choi, Y.D.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef] [Green Version]
- Ali, Q.; Ashraf, M. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: Growth, photosynthesis, water relations and oxidative defence mechanism. J. Agron. Crop Sci. 2011, 197, 258–271. [Google Scholar] [CrossRef]
- Schluepmann, H.; van Dijken, A.; Aghdasi, M.; Wobbes, B.; Paul, M.; Smeekens, S. Trehalose mediated growth inhibition of Arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiol. 2004, 135, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Avalbaev, A.M.; Somov, K.A.; Yuldashev, R.A.; Shakirova, F.M. Cytokinin oxidase is key enzyme of cytokinin degradation. Biochemistry 2012, 77, 1354–1361. [Google Scholar] [CrossRef]
- D’Aloia, M.; Bonhomme, D.; Bouché, F.; Tamseddak, K.; Ormenese, S.; Torti, S.; Coupland, G.; Périlleux, C. Cytokinin promotes flowering of Arabidopsis via transcriptional activation of the FT paralogue TSF. Plant J. 2011, 65, 972–979. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, C.; Mullins, M.G. Physiology of flowering in the grapevine—A Review. Am. J. Enol. Vitic. 1981, 32, 47–63. [Google Scholar]
- Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef]
- Fujita, K.; Aoki, Y.; Suzuki, S. Antidiabetic effects of novel cell culture established from grapevine, Vitis vinifera cv. Koshu. Cytotechnology 2018, 70, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokotsuka, K.; Nagao, A.; Nakazawa, K.; Sato, M. Changes in anthocyanins in berry skins of Merlot and Cabernet Sauvignon grapes grown in two soils modified with limestone or oyster shell versus a native soil over two years. Am. J. Enol. Vitic. 1999, 50, 1–12. [Google Scholar]
- Bakker, J.; Preston, N.W.; Timberlake, C.F. The determination of anthocyanins in aging red wines: Comparison of HPLC and spectral methods. Am. J. Enol. Vitic. 1986, 37, 121–126. [Google Scholar]
- Goto-Yamamoto, N.; Sawler, J.; Myles, S. Genetic analysis of East Asian grape cultivars suggests hybridization with wild Vitis. PLoS ONE 2015, 10, e0140841. [Google Scholar] [CrossRef] [Green Version]
- Candy, D.J.; Kilby, B.A. The biosynthesis of trehalose in the locust fat body. Biochem. J. 1961, 78, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Bartrina, I.; Otto, E.; Strnad, M.; Werner, T.; Schmülling, T. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 2011, 23, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Noriega, X.; Pérez, F.J. ABA biosynthesis genes are down-regulated while auxin and cytokinin biosynthesis genes are up-regulated during the release of grapevine buds from endodormancy. J. Plant Growth Regul. 2017, 36, 814–823. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, C.; Ma, J.Q.; Zhang, L.Y.; Yang, B.; Tang, X.Y.; Huang, L.; Zhou, X.T.; Lu, K.; Li, J.N. Genome-wide identification and expression profiling of cytokinin oxidase/dehydrogenase (CKX) genes reveal likely roles in pod development and stress responses in oilseed rape (Brassica napus L.). Genes 2018, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Brugière, N.; Jiao, S.; Hantke, S.; Zinselmeier, C.; Roessler, J.A.; Niu, X.; Jones, R.J.; Habben, J.E. Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, abscisic acid, and abiotic stress. Plant Physiol. 2003, 132, 1228–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin-cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Independent Variable | Partial Regression Coefficient | Standard Error | Standardized Partial Regression Coefficient | Tests of Significance of Partial Regression Coefficient | ||
|---|---|---|---|---|---|---|
| F-Value | t-Value | p-Value | ||||
| VvCKX5 expression | –1247.0 | 60.242 | –0.9977 | 428.48 | –20.7 | 0.0023 |
| VvCKX7 expression | –1218.0 | 695.20 | –0.7781 | 3.0696 | –1.752 | 0.2219 |
| VvCKX9 expression | –1824.9 | 536.71 | –0.9233 | 11.562 | –3.4 | 0.0767 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriyama, A.; Yamaguchi, C.; Enoki, S.; Aoki, Y.; Suzuki, S. Crosstalk Pathway between Trehalose Metabolism and Cytokinin Degradation for the Determination of the Number of Berries per Bunch in Grapes. Cells 2020, 9, 2378. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9112378
Moriyama A, Yamaguchi C, Enoki S, Aoki Y, Suzuki S. Crosstalk Pathway between Trehalose Metabolism and Cytokinin Degradation for the Determination of the Number of Berries per Bunch in Grapes. Cells. 2020; 9(11):2378. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9112378
Chicago/Turabian StyleMoriyama, Ayane, Chiho Yamaguchi, Shinichi Enoki, Yoshinao Aoki, and Shunji Suzuki. 2020. "Crosstalk Pathway between Trehalose Metabolism and Cytokinin Degradation for the Determination of the Number of Berries per Bunch in Grapes" Cells 9, no. 11: 2378. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9112378