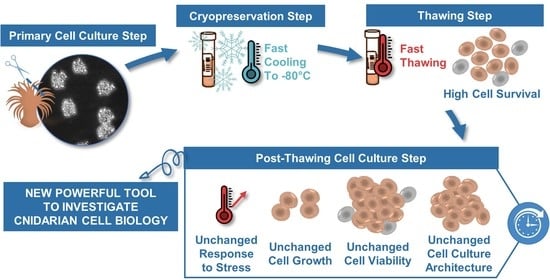
Cnidarian Cell Cryopreservation: A Powerful Tool for Cultivation and Functional Assays
Abstract
:1. Introduction
2. Material and Methods
2.1. Biological Material
2.2. Primary Cell Cultures
2.3. Cryopreservation Protocol
2.4. Cell Survival, Cell Viability, Cell Growth Rate, and Cell Size Assessment
2.5. Hyperthermal Stress Experiment
2.6. Statistical Analyses
3. Results and Discussion
3.1. Success in Set Up of Cryopreservation Protocol on Cell Survival
3.2. Absence of Cryopreservation Impact on Cell Recovery and Functional Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nandi, S. Animal Cell Culture and Virology; New India Publishing: Delhi, India, 2009; ISBN 978-93-80235-05-9. [Google Scholar]
- Maramorosch, K.; Sato, G.H. Advances in Cell Culture; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-1-4832-1506-8. [Google Scholar]
- Hall, B.K.; Moody, S.A. Cells in Evolutionary Biology: Translating Genotypes into Phenotypes—Past, Present, Future; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-65202-5. [Google Scholar]
- Vallone, D.; Santoriello, C.; Gondi, S.B.; Foulkes, N.S. Basic Protocols for Zebrafish Cell Lines. In Circadian Rhythms: Methods and Protocols; Rosato, E., Ed.; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, USA, 2007; pp. 429–441. ISBN 978-1-59745-257-1. [Google Scholar]
- Lynn, D.E. Novel techniques to establish new insect cell lines. Vitr. Cell. Dev. Biol. Anim. 2001, 37, 319–321. [Google Scholar] [CrossRef]
- Rinkevich, B. Marine Invertebrate Cell Cultures: New Millennium Trends. Mar. Biotechnol. 2005, 7, 429–439. [Google Scholar] [CrossRef]
- Rinkevich, B. Cell Cultures from Marine Invertebrates: New Insights for Capturing Endless Stemness. Mar. Biotechnol. 2011, 13, 345–354. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, Y. Marine invertebrate cell culture: A decade of development. J. Oceanogr. 2014, 70, 405–414. [Google Scholar] [CrossRef]
- Barnay-Verdier, S.; Dall’osso, D.; Joli, N.; Olivré, J.; Priouzeau, F.; Zamoum, T.; Merle, P.-L.; Furla, P. Establishment of primary cell culture from the temperate symbiotic cnidarian, Anemonia viridis. Cytotechnology 2013, 65, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Huete-Stauffer, C.; Valisano, L.; Gaino, E.; Vezzulli, L.; Cerrano, C. Development of long-term primary cell aggregates from Mediterranean octocorals. Vitr. Cell. Dev. Biol. Anim. 2015, 51, 815–826. [Google Scholar] [CrossRef]
- Lecointe, A.; Cohen, S.; Gèze, M.; Djediat, C.; Meibom, A.; Domart-Coulon, I. Scleractinian coral cell proliferation is reduced in primary culture of suspended multicellular aggregates compared to polyps. Cytotechnology 2013, 65, 705–724. [Google Scholar] [CrossRef] [Green Version]
- Mercurio, S.; Di Benedetto, C.; Sugni, M.; Candia Carnevali, M.D. Primary cell cultures from sea urchin ovaries: A new experimental tool. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 139–145. [Google Scholar] [CrossRef]
- Rabinowitz, C.; Moiseeva, E.; Rinkevich, B. In vitro cultures of ectodermal monolayers from the model sea anemone Nematostella vectensis. Cell Tissue Res. 2016, 366, 693–705. [Google Scholar] [CrossRef]
- Vandepas, L.E.; Warren, K.J.; Amemiya, C.T.; Browne, W.E. Establishing and maintaining primary cell cultures derived from the ctenophore Mnemiopsis leidyi. J. Exp. Biol. 2017, 220, 1197–1201. [Google Scholar] [CrossRef] [Green Version]
- Ventura, P.; Toullec, G.; Fricano, C.; Chapron, L.; Meunier, V.; Röttinger, E.; Furla, P.; Barnay-Verdier, S. Cnidarian Primary Cell Culture as a Tool to Investigate the Effect of Thermal Stress at Cellular Level. Mar. Biotechnol. 2018, 20, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F.; Oldenhof, H. (Eds.) Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1257, ISBN 978-1-4939-2192-8. [Google Scholar]
- Odintsova, N.A.; Boroda, A.V. Cryopreservation of the cells and larvae of marine organisms. Russ. J. Mar. Biol. 2012, 38, 101–111. [Google Scholar] [CrossRef]
- Paredes, E. Exploring the evolution of marine invertebrate cryopreservation – Landmarks, state of the art and future lines of research. Cryobiology 2015, 71, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, M.; Farrell, A.; Carter, V.L. Cryobiology of coral fragments. Cryobiology 2013, 66, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Mussino, F.; Pozzolini, M.; Valisano, L.; Cerrano, C.; Benatti, U.; Giovine, M. Primmorphs Cryopreservation: A New Method for Long-Time Storage of Sponge Cells. Mar. Biotechnol. 2012, 15. [Google Scholar] [CrossRef] [PubMed]
- Feuillassier, L.; Masanet, P.; Romans, P.; Barthélémy, D.; Engelmann, F. Towards a vitrification-based cryopreservation protocol for the coral Pocillopora damicornis L.: Tolerance of tissue balls to 4.5 M cryoprotectant solutions. Cryobiology 2015, 71, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Le Marrec-Croq, F.; Fritayre, P.; Chesné, C.; Guillouzo, A.; Dorange, G. Cryopreservation of Pecten maximus Heart Cells. Cryobiology 1998, 37, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.C.; La Peyre, J.F.; Buchanan, J.T.; Tiersch, T.R.; Cooper, R.K. Cryopreservation of heart cells from the eastern oyster. Vitr. Cell. Dev. Biol. Anim. 2001, 37, 237–243. [Google Scholar] [CrossRef]
- Odintsova, N.; Kiselev, K.; Sanina, N.; Kostetsky, E. Cryopreservation of primary cell cultures of marine invertebrates. CryoLetters 2001, 22, 299–310. [Google Scholar]
- Poncet, J.-M.; Serpentini, A.; Boucaud-Camou, E.; Lebel, J.-M. Cryopreservation of mantle dissociated cells from Haliotis tuberculata (Gastropoda) and postthawed primary cell cultures. Cryobiology 2002, 44, 38–45. [Google Scholar] [CrossRef]
- Hanquet-Dufour, A.C.; Kellner, K.; Heude, C.; Naimi, A.; Mathieu, M.; Poncet, J.M. Cryopreservation of Crassostrea gigas vesicular cells: Viability and metabolic activity. Cryobiology 2006, 53, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Kostetsky, E.Y.; Boroda, A.V.; Odintsova, N.A. Changes in the lipid composition of mussel (Mytilus trossulus) embryo cells during cryopreservation. Biophysics 2008, 53, 299–303. [Google Scholar] [CrossRef]
- Odintsova, N.A.; Boroda, A.V.; Velansky, P.V.; Kostetsky, E.Y. The fatty acid profile changes in marine invertebrate larval cells during cryopreservation. Cryobiology 2009, 59, 335–343. [Google Scholar] [CrossRef]
- Daugavet, M.A.; Blinova, M.I. Culture of mussel (Mytiuls edulis L.) mantle cells. Cell Tiss. Biol. 2015, 9, 233–243. [Google Scholar] [CrossRef]
- Dessai, S.N. Cryopreservation of cultured mantle cells of Paphia malabarica for perennial availability. Cryobiology 2018, 82, 93–98. [Google Scholar] [CrossRef]
- Pomponi, S.A.; Willoughby, R.; Kaighn, M.E.; Wright, A.E. Development of techniques for in vitro production of bioactive natural products from marine sponges. In InvertebrateCell Culture: Novel Directions and Biotechnology Applications; Maramorosch, K., Mitsuhashi, J., Eds.; Science Publishers, Inc.: Enfield, NH, USA, 1997; pp. 231–237. [Google Scholar]
- Pomponi, S.A.; Willoughby, R. Development of sponge cell cultures for biomedical application. In Aquatic Invertebrate Cell Culture; Springer: London, UK, 2000; pp. 323–336. [Google Scholar]
- Munroe, S.; Martens, D.E.; Sipkema, D.; Pomponi, S.A. Comparison of Cryopreservation Techniques For Cells of the Marine Sponge Dysidea Etheria. Available online: https://0-www-ingentaconnect-com.brum.beds.ac.uk/content/cryo/cryo/2018/00000039/00000004/art00007 (accessed on 19 December 2019).
- Conkling, M.; Hesp, K.; Munroe, S.; Sandoval, K.; Martens, D.E.; Sipkema, D.; Wijffels, R.H.; Pomponi, S.A. Breakthrough in Marine Invertebrate Cell Culture: Sponge Cells Divide Rapidly in Improved Nutrient Medium. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hagedorn, M.; Carter, V.; Martorana, K.; Paresa, M.K.; Acker, J.; Baums, I.B.; Borneman, E.; Brittsan, M.; Byers, M.; Henley, M.; et al. Preserving and Using Germplasm and Dissociated Embryonic Cells for Conserving Caribbean and Pacific Coral. PLoS ONE 2012, 7, e33354. [Google Scholar] [CrossRef] [Green Version]
- Hagedorn, M.; van Oppen, M.J.H.; Carter, V.; Henley, M.; Abrego, D.; Puill-Stephan, E.; Negri, A.; Heyward, A.; MacFarlane, D.; Spindler, R. First frozen repository for the Great Barrier Reef coral created. Cryobiology 2012, 65, 157–158. [Google Scholar] [CrossRef]
- Stolzing, A.; Naaldijk, Y.; Fedorova, V.; Sethe, S. Hydroxyethylstarch in cryopreservation – Mechanisms, benefits and problems. Transfus. Apher. Sci. 2012, 46, 137–147. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Song, J.; Pas, J.; Meijer, L.H.H.; Han, S. DMSO Induces Dehydration near Lipid Membrane Surfaces. Biophys. J. 2015, 109, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Fahy, G.M. The relevance of cryoprotectant “toxicity” to cryobiology. Cryobiology 1986, 23, 1–13. [Google Scholar] [CrossRef]
- Song, Y.C.; Khirabadi, B.S.; Lightfoot, F.; Brockbank, K.G.M.; Taylor, M.J. Vitreous cryopreservation maintains the function of vascular grafts. Nat. Biotechnol. 2000, 18, 296–299. [Google Scholar] [CrossRef]
- Elmoazzen, H.Y.; Poovadan, A.; Law, G.K.; Elliott, J.A.W.; McGann, L.E.; Jomha, N.M. Dimethyl sulfoxide toxicity kinetics in intact articular cartilage. Cell Tissue Bank. 2006, 8, 125. [Google Scholar] [CrossRef]
- Schaefer, V.W.; Dicke, K.A. Preservation of haemopoietic stem cells, transplantation potential and CFU-c activity of frozen marrow tested in mice, monkeys and man. In Cryopreservation of Normal and Neoplastic Cells; Weiner, R.S., Oldham, R.K., Schwartzenberg, L., Eds.; INSERM: Paris, France, 1973; pp. 63–69. [Google Scholar]
- Grilli, G.; Porcellini, A.; Lucarelli, G. Role of serum on cryopreservation and subsequent viability of mouse bone marrow hemopoietic stem cells. Cryobiology 1980, 17, 516–520. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Anchordoguy, T.; Carpenter, J.F.; Loomis, S.H.; Crowe, J.H. Mechanisms of interaction of amino acids with phospholipid bilayers during freezing. Biochim. Biophys. Acta (BBA) Biomembr. 1988, 946, 299–306. [Google Scholar] [CrossRef]
- Cabrita, E.; Anel, L.; Herraéz, M.P. Effect of external cryoprotectants as membrane stabilizers on cryopreserved rainbow trout sperm. Theriogenology 2001, 56, 623–635. [Google Scholar] [CrossRef]
- He, S.; Woods, L.C., III. Effects of dimethyl sulfoxide and glycine on cryopreservation induced damage of plasma membranes and mitochondria to striped bass (Morone saxatilis) sperm. Cryobiology 2004, 48, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Marco-Jiménez, F.; Garzón, D.L.; Peñaranda, D.S.; Pérez, L.; Viudes-de-Castro, M.P.; Vicente, J.S.; Jover, M.; Asturiano, J.F. Cryopreservation of European eel (Anguilla anguilla) spermatozoa: Effect of dilution ratio, foetal bovine serum supplementation, and cryoprotectants. Cryobiology 2006, 53, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.D. Modeling and Optimization of Cryopreservation. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F., Oldenhof, H., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; pp. 83–120. ISBN 978-1-4939-2193-5. [Google Scholar]
- Richier, S.; Rodriguez-Lanetty, M.; Schnitzler, C.E.; Weis, V.M. Response of the symbiotic cnidarian Anthopleura elegantissima transcriptome to temperature and UV increase. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2008, 3, 283–289. [Google Scholar] [CrossRef]
- Richier, S.; Sabourault, C.; Courtiade, J.; Zucchini, N.; Allemand, D.; Furla, P. Oxidative stress and apoptotic events during thermal stress in the symbiotic sea anemone, Anemonia viridis. FEBS J. 2006, 273, 4186–4198. [Google Scholar] [CrossRef]
- Moya, A.; Ganot, P.; Furla, P.; Sabourault, C. The transcriptomic response to thermal stress is immediate, transient and potentiated by ultraviolet radiation in the sea anemone Anemonia viridis. Mol. Ecol. 2012, 21, 1158–1174. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fricano, C.; Röttinger, E.; Furla, P.; Barnay-Verdier, S. Cnidarian Cell Cryopreservation: A Powerful Tool for Cultivation and Functional Assays. Cells 2020, 9, 2541. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9122541
Fricano C, Röttinger E, Furla P, Barnay-Verdier S. Cnidarian Cell Cryopreservation: A Powerful Tool for Cultivation and Functional Assays. Cells. 2020; 9(12):2541. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9122541
Chicago/Turabian StyleFricano, Clara, Eric Röttinger, Paola Furla, and Stéphanie Barnay-Verdier. 2020. "Cnidarian Cell Cryopreservation: A Powerful Tool for Cultivation and Functional Assays" Cells 9, no. 12: 2541. https://0-doi-org.brum.beds.ac.uk/10.3390/cells9122541