Electro-Oxidation of Humic Acids Using Platinum Electrodes: An Experimental Approach and Kinetic Modelling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Humic Acids Extraction
2.3. Electro-Oxidation Experiments
2.4. UV–Vis Analysis
2.5. Low-Pressure Size-Exclusion Chromatography (LP-SEC)
2.6. Total Organic Carbon
2.7. Elemental Analysis
3. Results and Discussion
3.1. Electro-Oxidation of HA
3.2. Kinetic Modelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef] [PubMed]
- Erto, A.; Andreozzi, R.; Di Natale, F.; Lancia, A.; Musmarra, D. Experimental and isotherm-models analysis on TCE and PCE adsorption onto activated carbon. Chem. Eng. Trans. 2009, 17, 293–298. [Google Scholar] [CrossRef]
- Colella, A.; de Gennaro, B.; Salvestrini, S.; Colella, C. Surface interaction of humic acids with natural and synthetic phillipsite. J. Porous Mater. 2015, 22, 501–509. [Google Scholar] [CrossRef]
- Jones, M.N.; Bryan, N.D. Colloidal properties of humic substances. Adv. Colloid Interface Sci. 1998, 78, 1–48. [Google Scholar] [CrossRef]
- Chianese, S.; Fenti, A.; Iovino, P.; Musmarra, D.; Salvestrini, S. Sorption of organic pollutants by humic acids: A review. Molecules 2020, 25, 918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, A.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Review on the electrochemical processes for the treatment of sanitary landfill leachates: Present and future. Appl. Catal. B Environ. 2015, 176, 183–200. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. HAzard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef]
- Schellekens, J.; Buurman, P.; Kalbitz, K.; Van Zomeren, A.; Vidal-Torrado, P.; Cerli, C.; Comans, R.N.J. Molecular Features of Humic Acids and Fulvic Acids from Contrasting Environments. Environ. Sci. Technol. 2017, 51, 1330–1339. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Lee, D.J.; Cheng, F. Treatment of domestic sewage with anoxic/oxic membrane-less microbial fuel cell with intermittent aeration. Bioresour. Technol. 2016, 218, 680–686. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Liu, W.; Ye, M.; Su, F. Effluent characteristics of advanced treatment for biotreated coking wastewater by electrochemical technology using BDD anodes. Environ. Sci. Pollut. Res. 2015, 22, 6827–6834. [Google Scholar] [CrossRef]
- Cowman, G.A.; Singer, P.C. Effect of bromide ion on haloacetic acid speciation resulting from chlorination and chloramination of aquatic humic substances. Environ. Sci. Technol. 1996, 30, 16–24. [Google Scholar] [CrossRef]
- Iovino, P.; Leone, V.; Salvestrini, S.; Capasso, S. Sorption of non-ionic organic pollutants onto immobilized humic acid. Desalin. Water Treat. 2015, 56, 55–62. [Google Scholar] [CrossRef]
- Lin, J.; Zhan, Y. Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites. Chem. Eng. J. 2012, 200, 202–213. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, S.; Ye, X.; Zhao, R.; Chen, D. Oxidation and coagulation removal of humic acid using Fenton process. Colloids Surf. A Physicochem. Eng. Asp. 2011, 379, 151–156. [Google Scholar] [CrossRef]
- Liu, J.; Cao, J.; Chen, H.; Zhou, D. Adsorptive removal of humic acid from aqueous solution by micro- and mesoporous covalent triazine-based framework. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 276–282. [Google Scholar] [CrossRef]
- Salvestrini, S. Diuron herbicide degradation catalyzed by low molecular weight humic acid-like compounds. Environ. Chem. Lett. 2013, 11, 359–363. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Yuan, X.; Jiang, L.; Xia, Q.; Chen, H. Photocatalytic removal of antibiotics from natural water matrices and swine wastewater via Cu(I) coordinately polymeric carbon nitride framework. Chem. Eng. J. 2020, 392, 123638. [Google Scholar] [CrossRef]
- Muhammad, A.; Shah, A.H.A.; Bilal, S. Effective Adsorption of Hexavalent Chromium and Divalent Nickel Ions from Water through Polyaniline, Iron Oxide, and Their Composites. Appl. Sci. 2020, 10, 2882. [Google Scholar] [CrossRef] [Green Version]
- Kamel, R.M.; Shahat, A.; Hegazy, W.H.; Khodier, E.M.; Awual, M.R. Efficient toxic nitrite monitoring and removal from aqueous media with ligand based conjugate materials. J. Mol. Liq. 2019, 285, 20–26. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Iqbal, J.; Islam, M.A.; Islam, A.; Khandaker, S.; Asiri, A.M.; Rahman, M.M. Ligand based sustainable composite material for sensitive nickel(II) capturing in aqueous media. J. Environ. Chem. Eng. 2020, 8, 103591. [Google Scholar] [CrossRef]
- Salvestrini, S. A modification of the Langmuir rate equation for diffusion-controlled adsorption kinetics. React. Kinet. Mech. Catal. 2019, 128, 571–586. [Google Scholar] [CrossRef]
- Leone, V.; Iovino, P.; Canzano, S.; Salvestrini, S.; Capasso, S. Water purification from humic acids by clinoptilolite-rich tuff. Environ. Eng. Manag. J. 2013, 12, 3–6. [Google Scholar]
- Salvestrini, S.; Jovanović, J.; Adnadjević, B. Comparison of adsorbent materials for herbicide diuron removal from water. Desalin. Water Treat. 2016, 57, 22868–22877. [Google Scholar] [CrossRef]
- Salvestrini, S.; Vanore, P.; Iovino, P.; Leone, V.; Capasso, S. Adsorption of simazine and boscalid onto acid-activated natural clinoptilolite. Environ. Eng. Manag. J. 2015, 14, 1705–1712. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Vemula, K.R.; Chang, Y.Y.; Yang, J.K.; Karri, R.R.; Koduru, J.R. Process optimization and modeling of lead removal using iron oxide nanocomposites generated from bio-waste mass. Chemosphere 2020, 243, 125257. [Google Scholar] [CrossRef] [PubMed]
- Spaltro, A.; Simonetti, S.; Laurella, S.; Ruiz, D.; Compañy, A.D.; Juan, A.; Allegretti, P. Adsorption of bentazone and imazapyc from water by using functionalized silica: Experimental and computational analysis. J. Contam. Hydrol. 2019, 227, 103542. [Google Scholar] [CrossRef]
- Czepa, W.; Pakulski, D.; Witomska, S.; Patroniak, V.; Ciesielski, A.; Samorì, P. Graphene oxide-mesoporous SiO2 hybrid composite for fast and efficient removal of organic cationic contaminants. Carbon 2020, 158, 193–201. [Google Scholar] [CrossRef]
- Yu, S.; Wang, J.; Cui, J. Preparation of a novel chitosan-based magnetic adsorbent CTS@SnO2@Fe3O4 for effective treatment of dye wastewater. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef]
- Canzano, S.; Capasso, S.; Di Natale, M.; Erto, A.; Iovino, P.; Musmarra, D. Remediation of groundwater polluted by aromatic compounds by means of adsorption. Sustainability 2014, 6, 4807–4822. [Google Scholar] [CrossRef] [Green Version]
- Erto, A.; Chianese, S.; Lancia, A.; Musmarra, D. On the mechanism of benzene and toluene adsorption in single-compound and binary systems: Energetic interactions and competitive effects. Desalin. Water Treat. 2017, 86, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Salvestrini, S.; Vanore, P.; Bogush, A.; Mayadevi, S.; Campos, L.C. Sorption of metaldehyde using granular activated carbon. J. Water Reuse Desalin. 2017, 7, 280–287. [Google Scholar] [CrossRef]
- Chu, L.; Zhang, J.; Wu, Z.; Wang, C.; Sun, Y.; Dong, S.; Sun, J. Solar-driven photocatalytic removal of organic pollutants over direct Z-scheme coral-branch shape Bi2O3/SnO2 composites. Mater. CHAract. 2020, 159, 110036. [Google Scholar] [CrossRef]
- Iovino, P.; Chianese, S.; Prisciandaro, M.; Musmarra, D. Triclosan photolysis: Operating condition study and photo-oxidation pathway. Chem. Eng. J. 2019, 377. [Google Scholar] [CrossRef]
- Iovino, P.; Chianese, S.; Canzano, S.; Prisciandaro, M.; Musmarra, D. Ibuprofen photodegradation in aqueous solutions. Environ. Sci. Pollut. Res. 2016, 23, 22993–23004. [Google Scholar] [CrossRef] [PubMed]
- Chianese, S.; Iovino, P.; Canzano, S.; Prisciandaro, M.; Musmarra, D. Ibuprofen degradation in aqueous solution by using UV light. Desalin. Water Treat. 2016, 57, 22878–22886. [Google Scholar] [CrossRef]
- Bensalah, N.; Ahmad, M.I.; Bedoui, A. Catalytic degradation of 4-ethylpyridine in water by heterogeneous photo-fenton process. Appl. Sci. 2019, 9, 5073. [Google Scholar] [CrossRef] [Green Version]
- Musmarra, D.; Prisciandaro, M.; Capocelli, M.; Karatza, D.; Iovino, P.; Canzano, S.; Lancia, A. Degradation of ibuprofen by hydrodynamic cavitation: Reaction pathways and effect of operational parameters. Ultrason. Sonochem. 2016, 29, 76–83. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Zhang, K.; Yao, R.H. Degradation of refractory pollutants by hydrodynamic cavitation: Key parameters to degradation rates. J. Hydrodyn. 2019, 31, 848–856. [Google Scholar] [CrossRef]
- Murray, C.A.; Parsons, S.A. Advanced oxidation processes: Flowsheet options for bulk natural organic matter removal. Water Sci. Technol. Water Supply 2004, 4, 113–119. [Google Scholar] [CrossRef]
- Silva, A.C.; Dezotti, M.; Sant’Anna, G.L. Treatment and detoxification of a sanitary landfill leachate. Chemosphere 2004, 55, 207–214. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rivas, F.J.; Gimeno, O. Comparison between photocatalytic ozonation and other oxidation processes for the removal of phenols from water. J. Chem. Technol. Biotechnol. 2005, 80, 973–984. [Google Scholar] [CrossRef]
- Zambrano, J.; Park, H.; Min, B. Enhancing electrochemical degradation of phenol at optimum pH condition with a Pt/Ti anode electrode. Environ. Technol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sopaj, F.; Rodrigo, M.A.; Oturan, N.; Podvorica, F.I.; Pinson, J.; Oturan, M.A. Influence of the anode materials on the electrochemical oxidation efficiency. Application to oxidative degradation of the pharmaceutical amoxicillin. Chem. Eng. J. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Särkkä, H.; Bhatnagar, A.; Sillanpää, M. Recent developments of electro-oxidation in water treatment—A review. J. Electroanal. Chem. 2015, 754, 46–56. [Google Scholar] [CrossRef]
- Qin, Y.; Cui, Y.; Lei, L.; Gao, Y.; Zhou, Z.; Li, Y.; Shi, X. An Electrochemical Process Comparison of As(III) in Simulated Groundwater at Low Voltage in Mixed and Divided Electrolytic Cells. Water 2020, 12, 1126. [Google Scholar] [CrossRef] [Green Version]
- Dao, K.C.; Yang, C.-C.; Chen, K.-F.; Tsai, Y.-P. Recent Trends in Removal Pharmaceuticals and Personal Care Products by Electrochemical Oxidation and Combined Systems. Water 2020, 12, 1043. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Mandal, P.; Dubey, B.K.; Gupta, A.K. Review on landfill leachate treatment by electrochemical oxidation: Drawbacks, challenges and future scope. Waste Manag. 2017, 69, 250–273. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, N.; Feng, C.; Chen, F.; Wang, H.; Kuang, P.; Feng, Z.; Liu, T.; Gao, Y.; Hu, W. Treatment of organic wastewater containing nitrogen and chlorine by combinatorial electrochemical system: Taking biologically treated landfill leachate treatment as an example. Chem. Eng. J. 2019, 364, 349–360. [Google Scholar] [CrossRef]
- Baddouh, A.; Bessegato, G.G.; Rguiti, M.M.; El Ibrahimi, B.; Bazzi, L.; Hilali, M.; Zanoni, M.V.B. Electrochemical decolorization of Rhodamine B dye: Influence of anode material, chloride concentration and current density. J. Environ. Chem. Eng. 2018, 6, 2041–2047. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.; Santos, D.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Electrochemical oxidation of humic acid and sanitary landfill leachate: Influence of anode material, chloride concentration and current density. Sci. Total Environ. 2016, 541, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Serrano, K.G. Indirect electrochemical oxidation using hydroxyl radical, active chlorine, and peroxodisulfate. In Electrochemical Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–164. ISBN 9780128131602. [Google Scholar] [CrossRef]
- Vagi, M.C.; Petsas, A.S. Recent advances on the removal of priority organochlorine and organophosphorus biorecalcitrant pesticides defied by Directive 2013/39/EU from environmental matrices by using advanced oxidation processes: An overview (2007–2018). J. Environ. Chem. Eng. 2019, 102940. [Google Scholar] [CrossRef]
- Zhu, X.; Ni, J.; Wei, J.; Xing, X.; Li, H. Destination of organic pollutants during electrochemical oxidation of biologically-pretreated dye wastewater using boron-doped diamond anode. J. HAzard. Mater. 2011, 189, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, R.; Quijada, C.; La Rosa-Toro, A.; Morallón, E. Electro-oxidation of cyanide on active and non-active anodes: Designing the electrocatalytic response of cobalt spinels. Sep. Purif. Technol. 2019, 208, 42–50. [Google Scholar] [CrossRef]
- Zhu, X.; Ni, J.; Lai, P. Advanced treatment of biologically pretreated coking wastewater by electrochemical oxidation using boron-doped diamond electrodes. Water Res. 2009, 43, 4347–4355. [Google Scholar] [CrossRef]
- Cañizares, P.; Martínez, L.; Paz, R.; Sáez, C.; Lobato, J.; Rodrigo, M.A. Treatment of Fenton-refractory olive oil mill wastes by electrochemical oxidation with boron-doped diamond anodes. J. Chem. Technol. Biotechnol. 2006, 81, 1331–1337. [Google Scholar] [CrossRef]
- Dbira, S.; Bensalah, N.; Ahmad, M.I.; Bedoui, A. Electrochemical oxidation/disinfection of urine wastewaters with different anode materials. Materials 2019, 12, 1254. [Google Scholar] [CrossRef] [Green Version]
- Trellu, C.; Péchaud, Y.; Oturan, N.; Mousset, E.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Comparative study on the removal of humic acids from drinking water by anodic oxidation and electro-Fenton processes: Mineralization efficiency and modelling. Appl. Catal. B Environ. 2016, 194, 32–41. [Google Scholar] [CrossRef]
- Capasso, S.; Salvestrini, S.; Roviello, V.; Trifuoggi, M.; Iovino, P. Electrochemical removal of humic acids from water using aluminum anode: Influence of chloride ion and current parameters. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Mahvi, A.H.; Bazrafshan, E.; Biglari, H. Humic acid removal from aqueous environments by electrocoagulation process using iron electrodes. E J. Chem. 2012, 9, 2453–2461. [Google Scholar] [CrossRef]
- Leone, V.; Musmarra, D.; Iovino, P.; Capasso, S. Sorption Equilibrium of Aromatic Pollutants onto Dissolved Humic Acids. Water. Air. Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Salvestrini, S. New insights into the interaction mechanism of humic acids with phillipsite. React. Kinet. Mech. Catal. 2017, 120, 735–752. [Google Scholar] [CrossRef]
- Engebretson, R.R.; von Wandruszka, R. Microorganization in Dissolved Humic Acids. Environ. Sci. Technol. 1994, 28, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Guetzloff, T.F.; Rice, J.A. Does humic acid form a micelle? Sci. Total Environ. 1994, 152, 31–35. [Google Scholar] [CrossRef]
- Mousset, E.; Dionysiou, D.D. Photoelectrochemical reactors for treatment of water and wastewater: A review. Environ. Chem. Lett. 2020, 18, 1301–1318. [Google Scholar] [CrossRef]
- Zazou, H.; Oturan, N.; Zhang, H.; Hamdani, M.; Oturan, M.A. Comparative study of electrochemical oxidation of herbicide 2,4,5-T: Kinetics, parametric optimization and mineralization pathway. Sustain. Environ. Res. 2017, 27, 15–23. [Google Scholar] [CrossRef]
- Seiffert, S.; Sprakel, J. Physical chemistry of supramolecular polymer networks. Chem. Soc. Rev. 2012, 41, 909–930. [Google Scholar] [CrossRef]
- Baalousha, M.; Motelica-Heino, M.; Coustumer, P. Le Conformation and size of humic substances: Effects of major cation concentration and type, pH, salinity, and residence time. Colloids Surf. A Physicochem. Eng. Asp. 2006, 272, 48–55. [Google Scholar] [CrossRef]
- Wang, L.F.; Wang, L.L.; Ye, X.D.; Li, W.W.; Ren, X.M.; Sheng, G.P.; Yu, H.Q.; Wang, X.K. Coagulation kinetics of humic aggregates in mono- and Di-valent electrolyte solutions. Environ. Sci. Technol. 2013, 47, 5042–5049. [Google Scholar] [CrossRef]
- Rodig, O.R. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1963; Volume 6, ISBN 978-0470616376. [Google Scholar]
- Anglada, Á.; Urtiaga, A.; Ortiz, I.; Mantzavinos, D.; Diamadopoulos, E. Boron-doped diamond anodic treatment of landfill leachate: Evaluation of operating variables and formation of oxidation by-products. Water Res. 2011, 45, 828–838. [Google Scholar] [CrossRef]
- Pérez, G.; Saiz, J.; Ibañez, R.; Urtiaga, A.M.; Ortiz, I. Assessment of the formation of inorganic oxidation by-products during the electrocatalytic treatment of ammonium from landfill leachates. Water Res. 2012, 46, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R. Solid phase sensitive palladium(II) ions detection and recovery using ligand based efficient conjugate nanomaterials. Chem. Eng. J. 2016, 300, 264–272. [Google Scholar] [CrossRef]
- Awual, M.R. New type mesoporous conjugate material for selective optical copper(II) ions monitoring & removal from polluted waters. Chem. Eng. J. 2017, 307, 85–94. [Google Scholar] [CrossRef]
- Awual, M.R. Ring size dependent crown ether based mesoporous adsorbent for high cesium adsorption from wastewater. Chem. Eng. J. 2016, 303, 539–546. [Google Scholar] [CrossRef]
- Cherney, D.P.; Duirk, S.E.; Tarr, J.C.; Collette, T.W. Monitoring the speciation of aqueous free chlorine from pH 1 to 12 with Raman spectroscopy to determine the identity of the potent low-pH oxidant. Appl. Spectrosc. 2006, 60, 764–772. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2010; ISBN 978-0-19-954337-3. [Google Scholar]

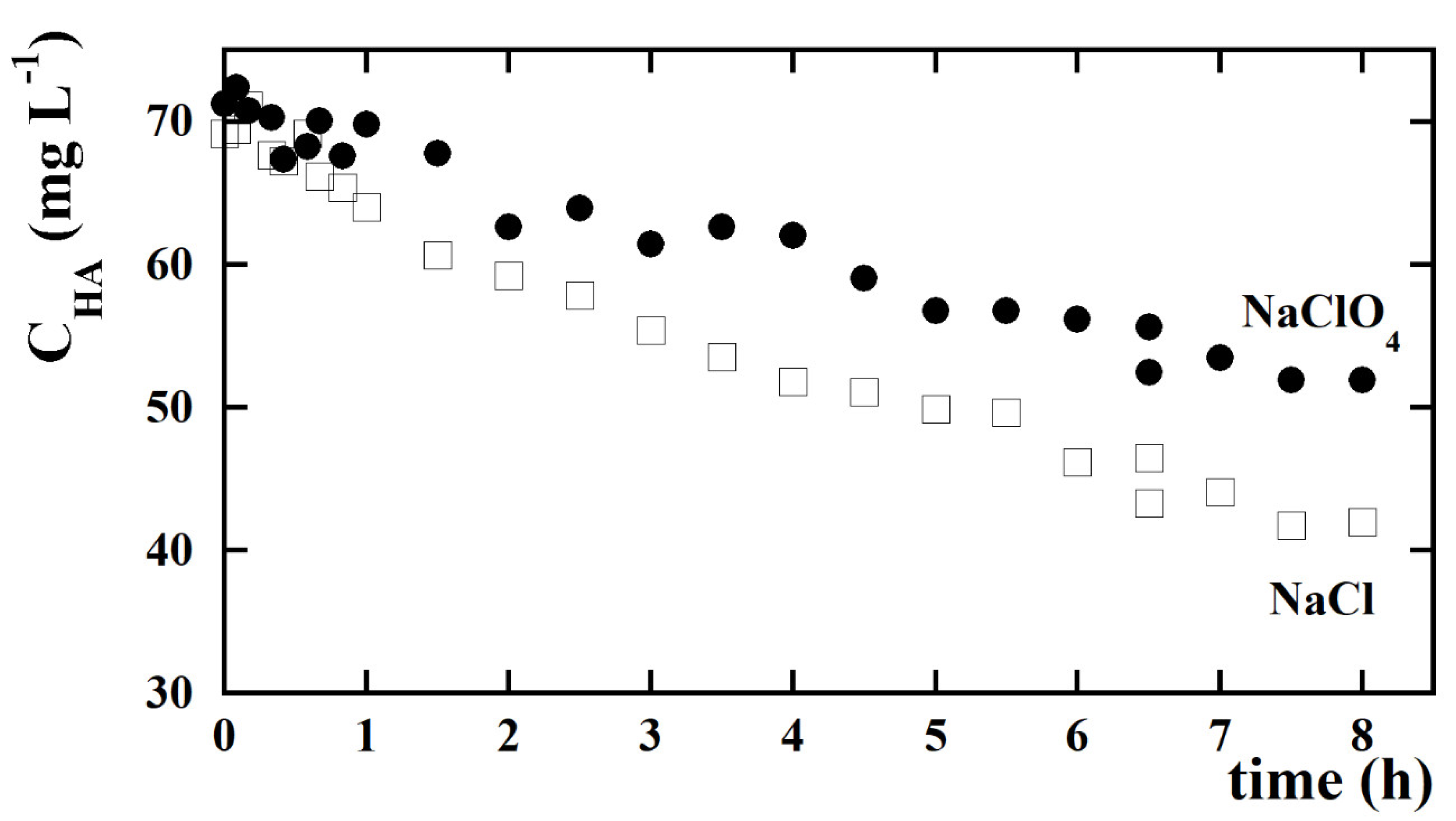

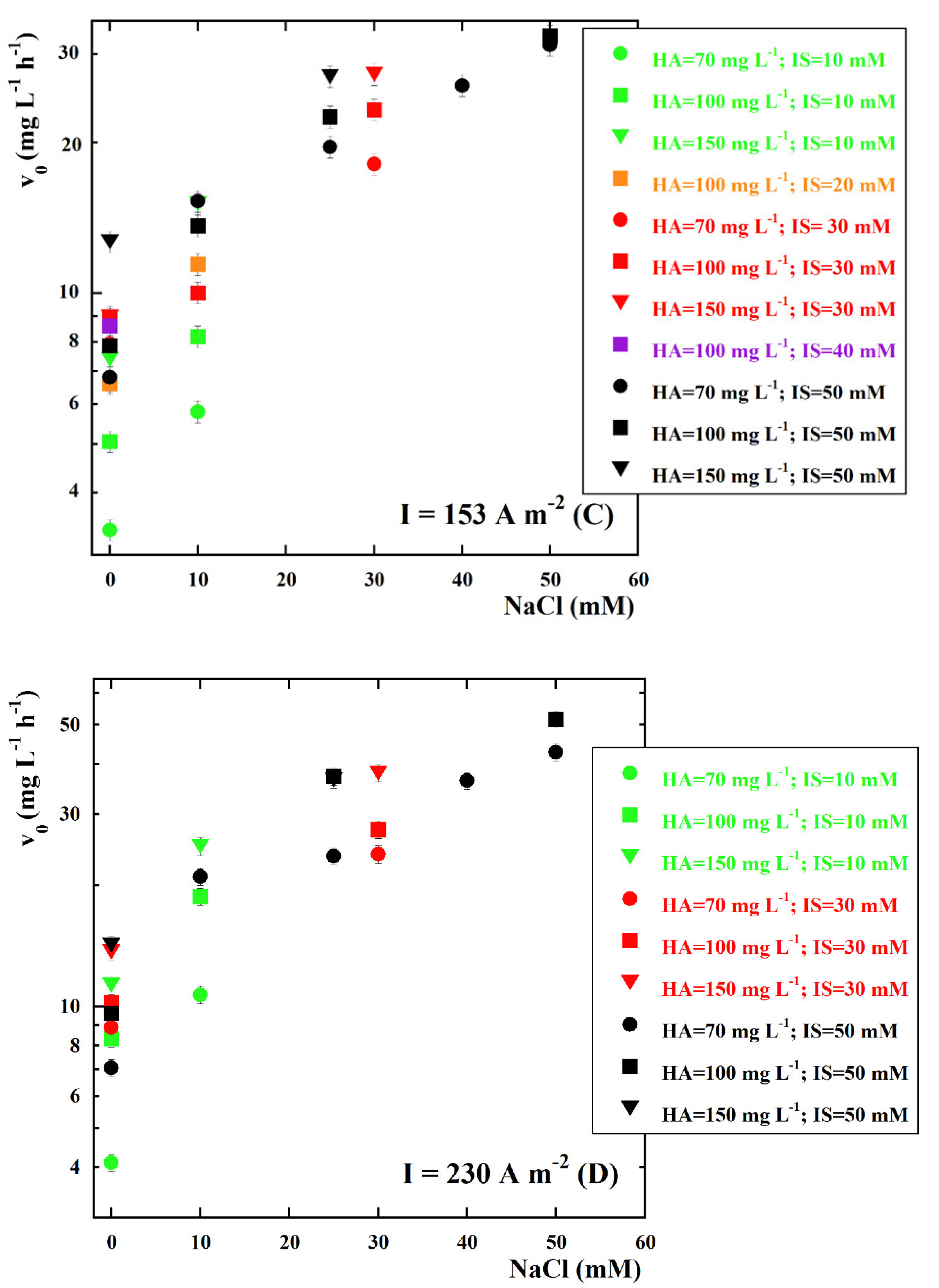
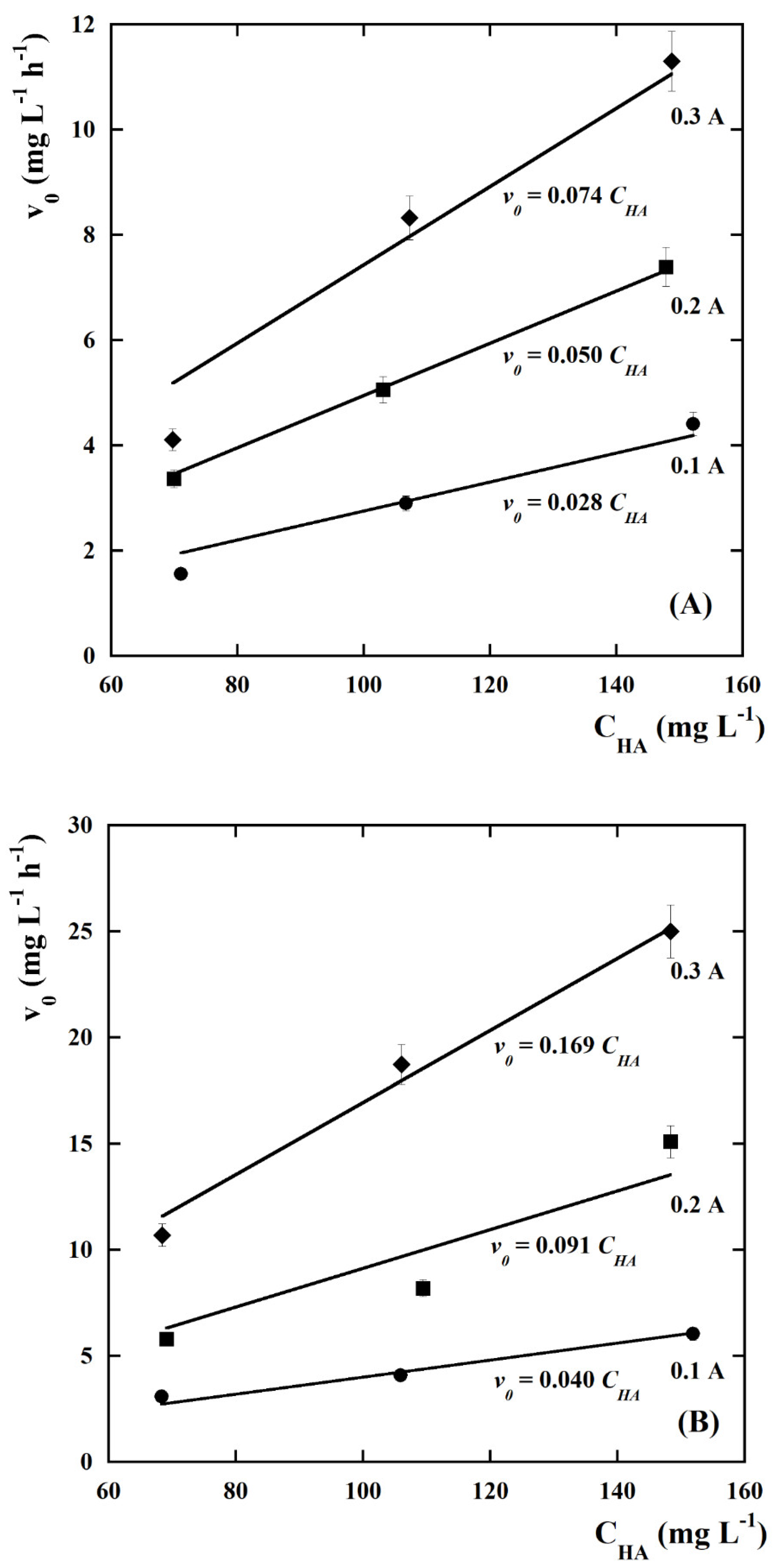
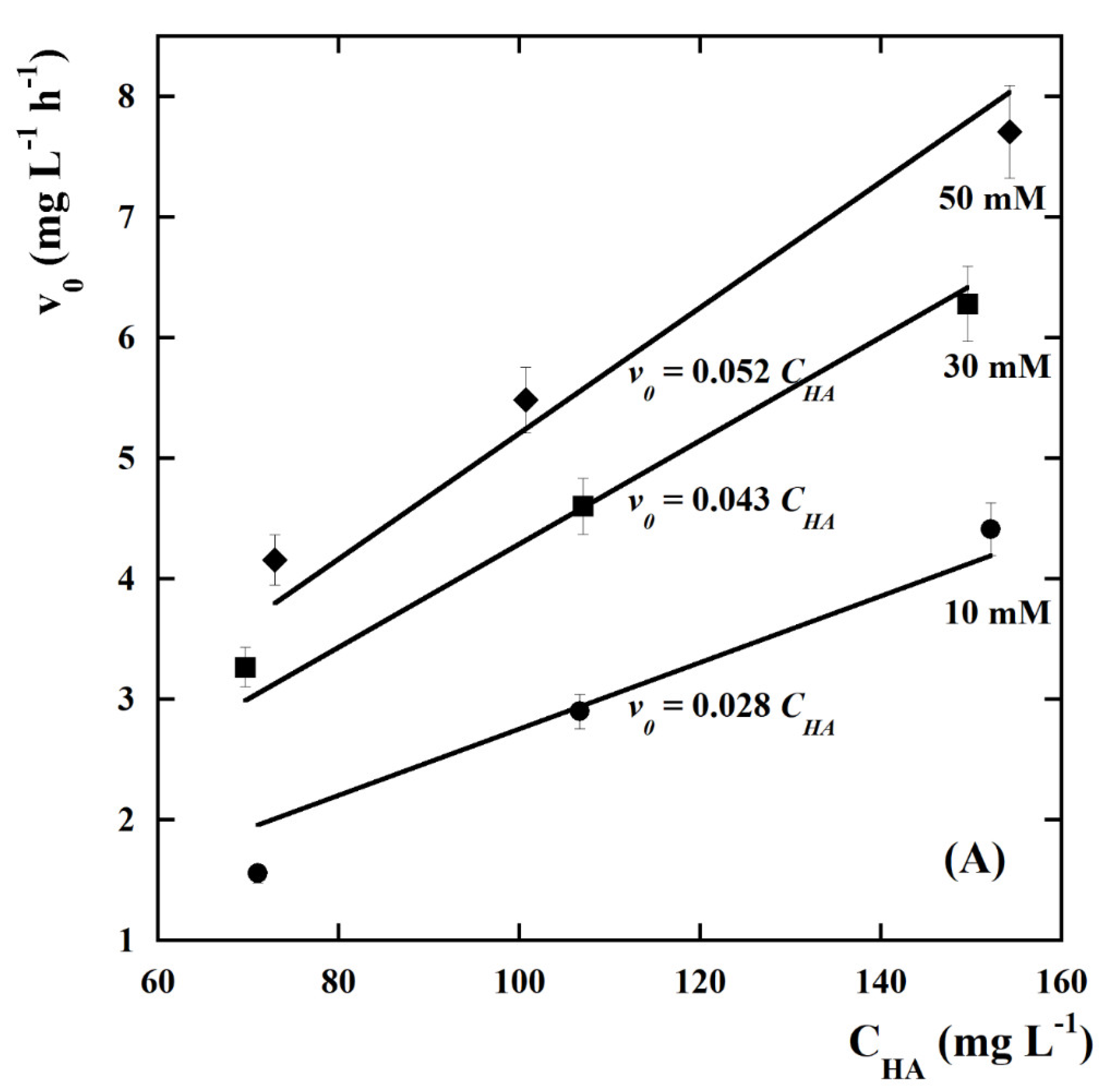
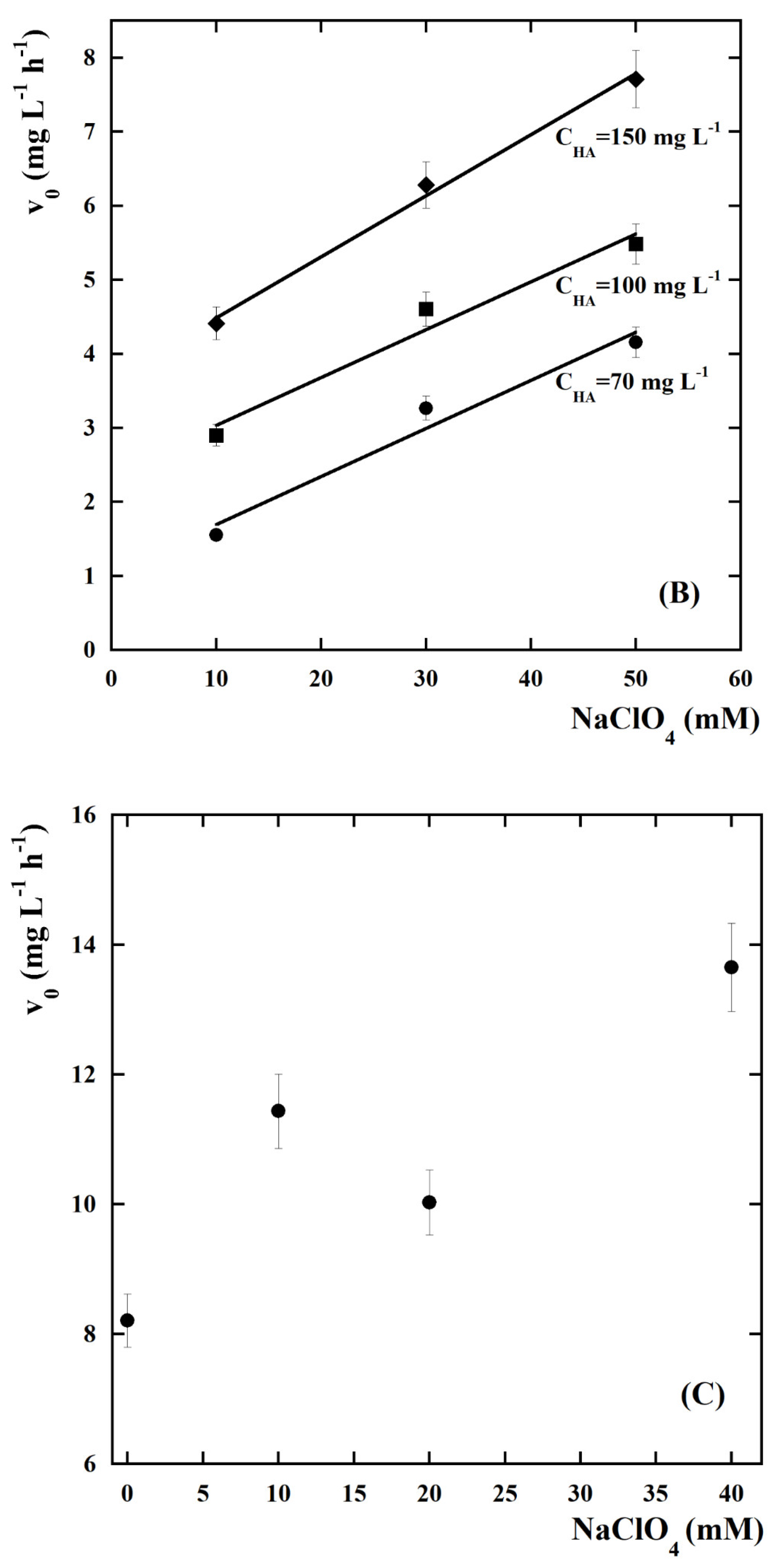
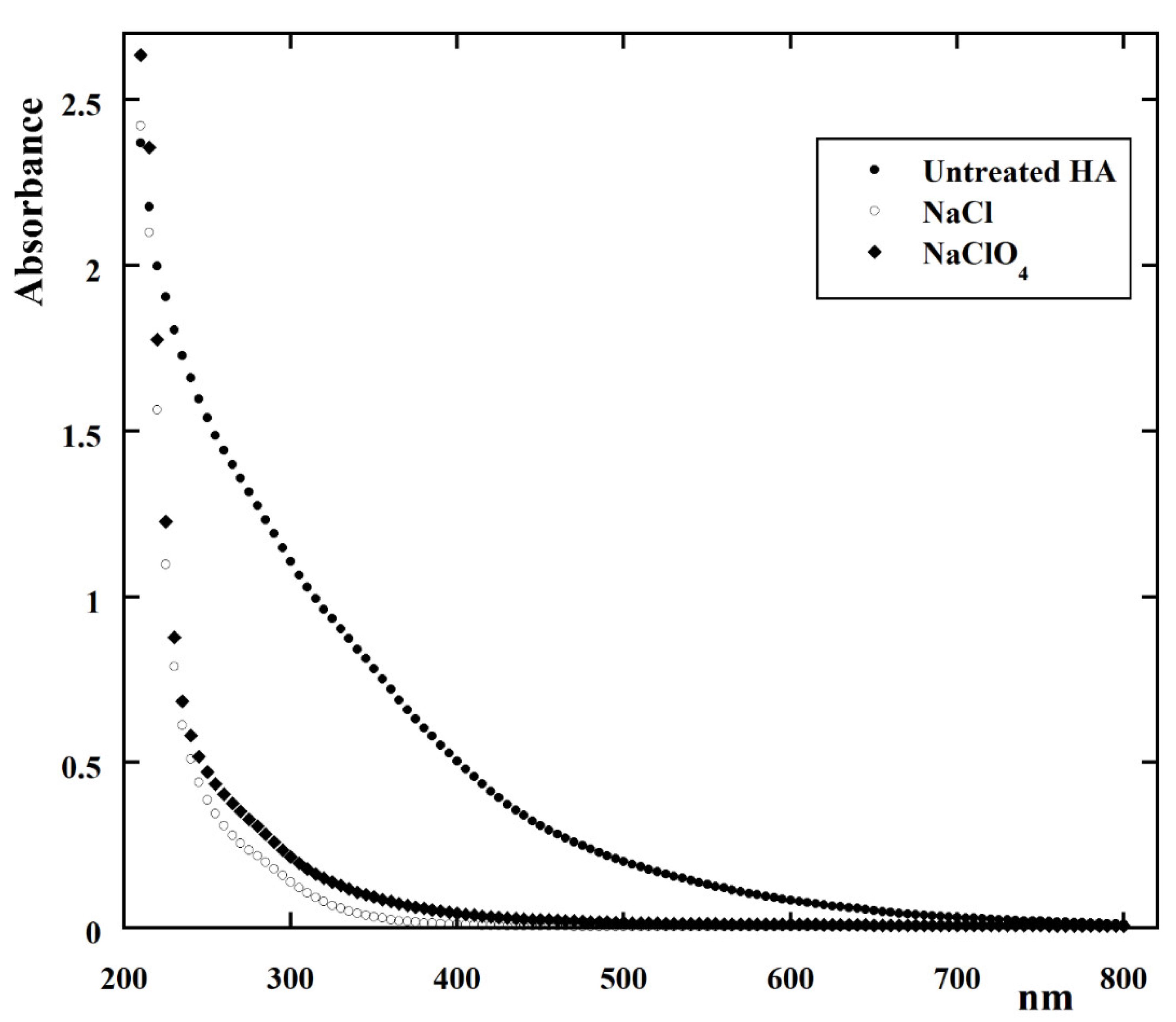
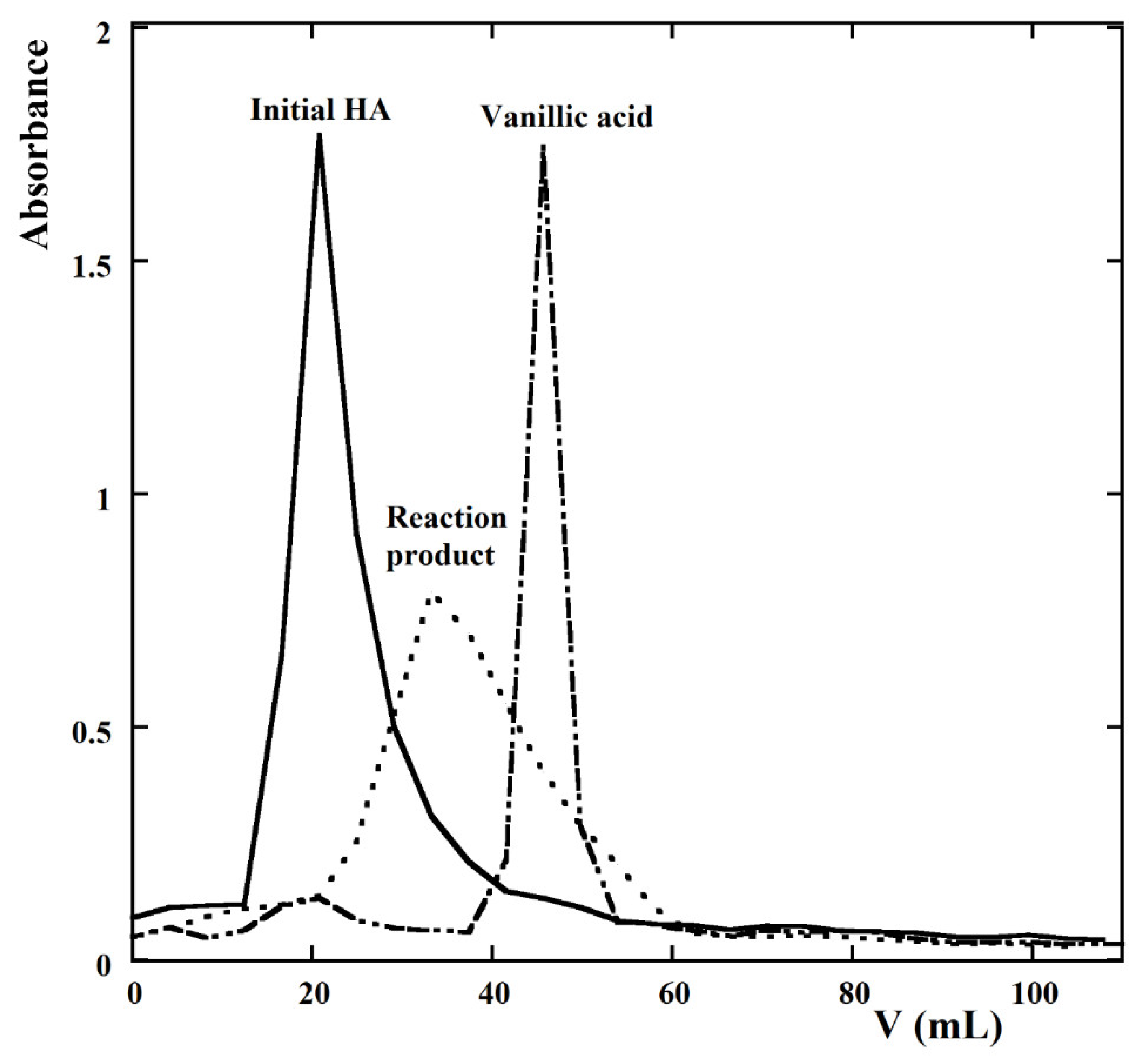
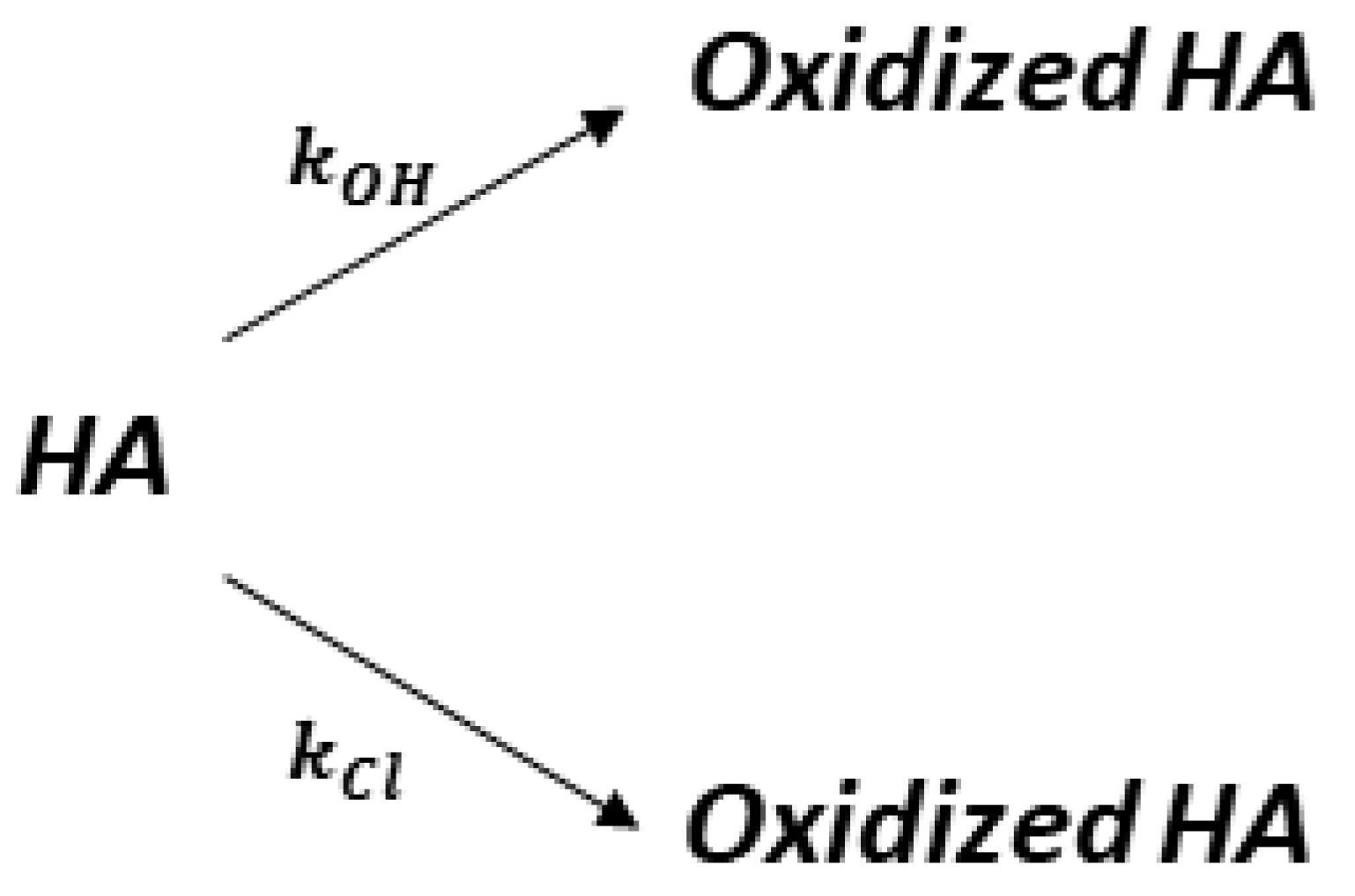

| kCl (Ln+1 m2 mmol−(n+1)h−1 A−1) | kOH (Ln m2 mmol−nh−1 A−1) | n | Adjusted R2 |
|---|---|---|---|
| (1.4 ± 0.3) × 10−5 | (2.0 ± 0.4) × 10−4 | 0.24 ± 0.06 | 0.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvestrini, S.; Fenti, A.; Chianese, S.; Iovino, P.; Musmarra, D. Electro-Oxidation of Humic Acids Using Platinum Electrodes: An Experimental Approach and Kinetic Modelling. Water 2020, 12, 2250. https://0-doi-org.brum.beds.ac.uk/10.3390/w12082250
Salvestrini S, Fenti A, Chianese S, Iovino P, Musmarra D. Electro-Oxidation of Humic Acids Using Platinum Electrodes: An Experimental Approach and Kinetic Modelling. Water. 2020; 12(8):2250. https://0-doi-org.brum.beds.ac.uk/10.3390/w12082250
Chicago/Turabian StyleSalvestrini, Stefano, Angelo Fenti, Simeone Chianese, Pasquale Iovino, and Dino Musmarra. 2020. "Electro-Oxidation of Humic Acids Using Platinum Electrodes: An Experimental Approach and Kinetic Modelling" Water 12, no. 8: 2250. https://0-doi-org.brum.beds.ac.uk/10.3390/w12082250





