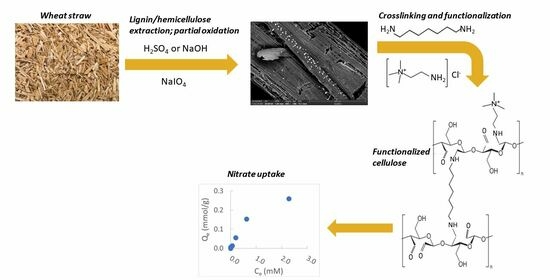
Nitrate Uptake by Cellulose-Based Anion Exchange Polymers Derived from Wheat Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Sources of Materials and Reagents
2.3. Acid or Base Pretreatment
| Mild Acid (ID 1) | Mild Base (ID 2) | High Acid | High Base (ID 3) | |
|---|---|---|---|---|
| Concentration of acid or base | 0.4% H2SO4 | 0.72% NaOH | 1% H2SO4 | 1% NaOH |
| Duration and temperature | 4 h at 60 °C | 1.5 h at 110 °C | 1.5 h at 120 °C | 1.5 h at 120 °C |
| Pressure | Atmospheric | Atmospheric | 15 psig | 15 psig |
| Ratio of acid or base to wheat straw | 12 mL per g wheat straw | 15 mL per g wheat straw | 20 mL per g wheat straw | 20 mL per g wheat straw |
| Polymer ID | Pretreatment | Temperature & Pressure | Periodate Concentration (M) | Oxidation Time (Days) | Cu# (g Cu/100 g Solid) (before Periodate Oxidation) | Cu# (g Cu/100 g Solid) (after Periodate Oxidation) | Aldehyde Concentration (µmol/g) (after Periodate Oxidation) |
|---|---|---|---|---|---|---|---|
| acid | mild | 0.031 | 1 | 0.706 ± 0.061 | 5.30 ± 0.49 | 87.1 ± 8.2 | |
| 1 | acid | mild | 0.031 | 10 | 0.706 ± 0.061 | 5.30 ± 0.18 | 87.1 ± 3.0 |
| acid | high | 0.12 | 1 | 1.48 ± 0.49 | 5.29 ± 0.18 | 87.0 ± 3.1 | |
| acid | high | 0.12 | 10 | 1.48 ± 0.49 | 4.56 ± 0.37 | 74.8 ± 6.1 | |
| base | mild | 0.031 | 1 | 0.211 ± 0.000 | 4.65 ± 0.96 | 76.4 ± 16.1 | |
| 2 | base | mild | 0.031 | 10 | 0.211 ± 0.000 | 5.58 ± 0.06 | 91.8 ± 1.0 |
| base | mild | 0.12 | 1 | 0.211 ± 0.000 | 5.51 ± 0.10 | 90.6 ± 1.7 | |
| base | high | 0.12 | 1 | 0.317 ± 0.001 | 5.40 ± 0.32 | 88.8 ± 5.0 | |
| 3 | base | high | 0.12 | 10 | 0.317 ± 0.001 | 6.30 ± 0.18 | 103.1 ± 2.9 |
2.4. Periodate Oxidation
2.5. Determination of the Copper Number
2.6. Crosslinking and Functionalization
2.7. Scanning Electron Microscopy
2.8. Nitrate Adsorption
3. Results
3.1. Polymer Characterization
3.2. Nitrate Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Spalding, R.F.; Exner, M.E. Occurrence of Nitrate in Groundwater—A Review. J. Environ. Qual. 1993, 22, 392–402. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpaa, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.B.; Darby, J.L.; Seidel, C.; Gorman, C. Nitrate in Potable Water Supplies: Alternative Management Strategies. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2203–2286. [Google Scholar] [CrossRef]
- EPA Drinking Water Contaminants—Standards and Regulations. Available online: https://www.epa.gov/dwstandardsregulations (accessed on 31 October 2019).
- World Health Organization Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; p. 397.
- Comly, H.H. Cyanosis in Infants Caused by Nitrates in Well Water. JAMA-J. Am. Med. Assoc. 1945, 129, 112–116. [Google Scholar] [CrossRef]
- Schaider, L.A.; Swetschinski, L.; Campbell, C.; Rudel, R.A. Environmental justice and drinking water quality: Are there socioeconomic disparities in nitrate levels in U.S. drinking water? Environ. Health 2019, 18, 15. [Google Scholar] [CrossRef]
- Balazs, C.; Morello-Frosch, R.; Hubbard, A.; Ray, I. Social Disparities in Nitrate-Contaminated Drinking Water in California’s San Joaquin Valley. Environ. Health Perspect. 2011, 119, 1272–1278. [Google Scholar] [CrossRef]
- Deen, A.; Estrada, T.; Everts, C.; Farina, S.; Gibler, J.; Guzman, M.; Levine, J.; Ramos, P.; Vanderwarker, A.; Ventura, A.; et al. Thirsty for Justice: A People’s Blueprint for California Water; The Environmental Justice Coalition for Water: Oakland, CA, USA, 2005. [Google Scholar]
- Allaire, M.; Wu, H.; Lall, U. National trends in drinking water quality violations. Proc. Natl. Acad. Sci. USA 2018, 115, 2078–2083. [Google Scholar] [CrossRef]
- Jensen, V.B.; Darby, J.L. Drinking Water Treatment for Nitrate, Technical Report 6, Addressing Nitrate in California’s Drinking Water With a Focus on Tulare Lake Basin and Salinas Valley Groundwater; Report for the State Water Resources Control Board Report to the Legislature; University of California: Davis, CA, USA, 2012; 182p. [Google Scholar]
- Moore, E.; Matalon, E.; Balazs, C.; Clary, J.; Firestone, L.; De Anda, S.; Guzman, M. The Human Costs of Nitrate-contaminated Drinking Water in the San Joaquin Valley; Pacific Institute: Oakland, CA, USA, 2011; 71p. [Google Scholar]
- Holl, W.H. Water Treatment|Anion Exchangers: Ion Exchange. In Encyclopedia of Separation Science, Wilson, I.D., Ed.; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Olin Corporation Epichlorohydrin: Product Stewardship Manual, Form No. 296-01301-0417PI. 2016. Available online: https://olinepoxy.com/wp-content/uploads/2017/05/Epichlorohydrin_Stewardship_Manual.pdf (accessed on 3 October 2019).
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), Seventh Revised Edition; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.J.; Gindulyte, A.; He, J.; He, S.Q.; Li, Q.L.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Shi, S.; Cao, Y.; Chen, M.; Zhou, X. Fast modification on wheat straw outer surface by water vapor plasma and its application on composite material. Sci. Rep. 2018, 8, 2279. [Google Scholar] [CrossRef]
- Passoth, V.; Sandgren, M. Biofuel production from straw hydrolysates: Current achievements and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5105–5116. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Eastham, S.D.; Liu, T.; Norford, L.K.; Barrett, S.R.H. Air quality impacts of crop residue burning in India and mitigation alternatives. Nat. Commun. 2022, 13, 6537. [Google Scholar] [CrossRef] [PubMed]
- Orlando, U.S.; Baes, A.U.; Nishijima, W.O.M. A new procedure to produce lignocellulosic anion exchangers from agricultural waste materials. Bioresour. Technol. 2002, 83, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Orlando, U.S.; Baes, A.U.; Nishijima, W.; Okada, M. Preparation of agricultural residue anion exchangers and its nitrate maximum adsorption capacity. Chemosphere 2002, 48, 1041–1046. [Google Scholar] [CrossRef]
- Yu, W.; Gao, B.Y.; Yue, W.W.; Yue, Q.Y. Preparation and utilization of wheat straw anionic sorbent for the removal of nitrate from aqueous solution. J. Environ. Sci. 2007, 19, 1305–1310. [Google Scholar] [CrossRef]
- Xu, X.; Gao, B.-Y.; Yue, Q.-Y.; Zhong, Q.-Q. Preparation of agricultural by-product based anion exchanger and its utilization for nitrate and phosphate removal. Bioresour. Technol. 2010, 101, 8558–8564. [Google Scholar] [CrossRef]
- Xing, X.; Gao, B.Y.; Zhong, Q.Q.; Yue, Q.Y.; Li, Q.A. Sorption of nitrate onto amine-crosslinked wheat straw: Characteristics, column sorption and desorption properties. J. Hazard. Mater. 2011, 186, 206–211. [Google Scholar] [CrossRef]
- Xu, X.; Gao, B.Y.; Yue, Q.Y.; Li, Q.; Wang, Y. Nitrate adsorption by multiple biomaterial based resins: Application of pilot-scale and lab-scale products. Chem. Eng. J. 2013, 234, 397–405. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, S.; Xie, M.; Christodoulatos, C.; Meng, X. Competitive adsorption of nitrate, phosphate, and sulfate on amine-modified wheat straw: In-situ infrared spectroscopic and density functional theory study. Environ. Res. 2022, 215, 114368. [Google Scholar] [CrossRef]
- Katal, R.; Baei, M.S.; Rahmati, H.T.; Esfandian, H. Kinetic, isotherm and thermodynamic study of nitrate adsorption from aqueous solution using modified rice husk. J. Ind. Eng. Chem. 2012, 18, 295–302. [Google Scholar] [CrossRef]
- Keranen, A.; Leiviska, T.; Gao, B.Y.; Hormi, O.; Tanskanen, J. Preparation of novel anion exchangers from pine sawdust and bark, spruce bark, birch bark and peat for the removal of nitrate. Chem. Eng. Sci. 2013, 98, 59–68. [Google Scholar] [CrossRef]
- Keranen, A.; Leiviska, T.; Hormi, O.; Tanskanen, J. Removal of nitrate by modified pine sawdust: Effects of temperature and co-existing anions. J. Environ. Manag. 2015, 147, 46–54. [Google Scholar] [CrossRef]
- Keranen, A.; Leiviska, T.; Hormi, O.; Tanskanen, J. Preparation of cationized pine sawdust for nitrate removal: Optimization of reaction conditions. J. Environ. Manag. 2015, 160, 105–112. [Google Scholar] [CrossRef]
- Song, W.; Gao, B.Y.; Xu, X.; Wang, F.; Xue, N.; Sun, S.L.; Song, W.C.; Jia, R.B. Adsorption of nitrate from aqueous solution by magnetic amine-crosslinked biopolymer based corn stalk and its chemical regeneration property. J. Hazard. Mater. 2016, 304, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Kalaruban, M.; Loganathan, P.; Shim, W.G.; Kandasamy, J.; Ngo, H.H.; Vigneswaran, S. Enhanced removal of nitrate from water using amine-grafted agricultural wastes. Sci. Total. Environ. 2016, 565, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Morooka, T.; Norimoto, M.; Yamada, T. Periodate-Oxidation of Cellulose by Homogeneous Reaction. J. Appl. Polym. Sci. 1989, 38, 849–858. [Google Scholar] [CrossRef]
- Kim, U.J.; Kuga, S. Thermal decomposition of dialdehyde cellulose and its nitrogen-containing derivatives. Thermochim. Acta 2001, 369, 79–85. [Google Scholar] [CrossRef]
- Wu, M.; Kuga, S.N. Cationization of cellulose fabrics by polyallylamine binding. J. Appl. Polym. Sci. 2006, 100, 1668–1672. [Google Scholar] [CrossRef]
- Dash, R. Synthesis and Characterization of Novel Cellulosics. Ph.D. Dissertation, Georgia Institute of Technology, North Avenue Atlanta, GA, USA, 2012. [Google Scholar]
- Visanko, M.; Liimatainen, H.; Sirvio, J.A.; Heiskanen, J.P.; Niinimaki, J.; Hormi, O. Amphiphilic Cellulose Nanocrystals from Acid-Free Oxidative Treatment: Physicochemical Characteristics and Use as an Oil-Water Stabilizer. Biomacromolecules 2014, 15, 2769–2775. [Google Scholar] [CrossRef]
- Samanta, A.K.; Kar, T.R.; Mukhopadhyay, A.; Shome, D.; Konar, A. Cationization of Periodate Oxidized Cotton Cellulose of Muslin Fabric Using Natural Amino Acid Extract from Soya Bean Seed Waste and Its Eco-Friendly Dyeing. J. Mater. Sci. Appl. 2015, 1, 142–154. [Google Scholar]
- Samanta, A.K.; Kar, T.R.; Mukhopadhyah, A.; Shome, D.; Konar, A. Studies on Dyeing Process Variables for Salt Free Reactive Dyeing of Glycine Modified Cationized Cotton Muslin Fabric. J. Inst. Eng. India Ser. E 2015, 96, 31–44. [Google Scholar] [CrossRef]
- Lindh, J.; Ruan, C.Q.; Stromme, M.; Mihranyan, A. Preparation of Porous Cellulose Beads via Introduction of Diamine Spacers. Langmuir 2016, 32, 5600–5607. [Google Scholar] [CrossRef]
- Sirvio, J.A.; Visanko, M.; Laitinen, O.; Ammala, A.; Liimatainen, H. Amino-modified cellulose nanocrystals with adjustable hydrophobicity from combined regioselective oxidation and reductive amination. Carbohydr. Polym. 2016, 136, 581–587. [Google Scholar] [CrossRef]
- Yang, H.; van de Ven, T.G.M. Preparation of hairy cationic nanocrystalline cellulose. Cellulose 2016, 23, 1791–1801. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Kamel, S.; Salama, A.; Youssef, M.A.; Teyor, W.E.; Tohamy, H.A.S. Amphiphilic Cellulose as Stabilizer for Oil/Water Emulsion. Egypt. J. Chem. 2017, 60, 181–204. [Google Scholar] [CrossRef]
- Park, S.; Choi, H.M. Microwave-Mediated Rapid Oxidation and Cationization of Cotton Cellulose. Cell Chem. Technol. 2018, 52, 311–322. [Google Scholar]
- Yan, G.H.; Zhang, X.Q.; Li, M.Z.; Zhao, X.Y.; Zeng, X.H.; Sun, Y.; Tang, X.; Lei, T.Z.; Lin, L. Stability of Soluble Dialdehyde Cellulose and the Formation of Hollow Microspheres: Optimization and Characterization. ACS Sustain. Chem. Eng. 2019, 7, 2151–2159. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development, A/RES/70/1; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Mehdinejadiani, B.; Amininasab, S.M.; Manhooei, L. Enhanced adsorption of nitrate from water by modified wheat straw: Equilibrium, kinetic and thermodynamic studies. Water Sci. Technol. 2019, 79, 302–313. [Google Scholar] [CrossRef]
- Govumoni, S.P.; Koti, S.; Kothagouni, S.Y.; Venkateshwar, S.; Linga, V.R. Evaluation of pretreatment methods for enzymatic saccharification of wheat straw for bioethanol production. Carbohydr. Polym. 2013, 91, 646–650. [Google Scholar] [CrossRef]
- Li, P.P.; Sirvio, J.A.; Asante, B.; Liimatainen, H. Recyclable deep eutectic solvent for the production of cationic nanocelluloses. Carbohydr. Polym. 2018, 199, 219–227. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Tucker, M.P.; Keller, F.A.; Beaty, D.A.; Connors, K.M.; Eddy, F.P. Dilute acid hydrolysis of softwoods. Appl. Biochem. Biotech. 1999, 77–79, 133–142. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Tucker, M.P.; Keller, F.A.; Eddy, F.P. Two-stage dilute-acid pretreatment of softwoods. Appl. Biochem. Biotech. 2000, 84–86, 561–576. [Google Scholar] [CrossRef]
- Chang, V.S.; Burr, B.; Holtzapple, M.T. Lime pretreatment of switchgrass. Appl. Biochem. Biotech. 1997, 63–65, 3–19. [Google Scholar] [CrossRef]
- Chang, V.S.; Nagwani, M.; Holtzapple, M.T. Lime pretreatment of crop residues bagasse and wheat straw. Appl. Biochem. Biotech. 1998, 74, 135–159. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kalra, K.L.; Grewal, H.S. Enzymatic saccharification of pretreated sunflower stalks. Biomass Bioenerg. 2002, 23, 237–243. [Google Scholar] [CrossRef]
- Meng, X.Z.; Wells, T.; Sun, Q.N.; Huang, F.; Ragauskas, A. Insights into the effect of dilute acid, hot water or alkaline pretreatment on the cellulose accessible surface area and the overall porosity of Populus. Green. Chem. 2015, 17, 4239–4246. [Google Scholar] [CrossRef]
- Calvini, P.; Gorassini, A.; Luciano, G.; Franceschi, E. FTIR and WAXS analysis of periodate oxycellulose: Evidence for a cluster mechanism of oxidation. Vib. Spectrosc. 2006, 40, 177–183. [Google Scholar] [CrossRef]
- Lindh, J.; Carlsson, D.O.; Stromme, M.; Mihranyan, A. Convenient One-Pot Formation of 2,3-Dialdehyde Cellulose Beads via Periodate Oxidation of Cellulose in Water. Biomacromolecules 2014, 15, 1928–1932. [Google Scholar] [CrossRef]
- ASTM D919-97; Standard Test Method for Copper Number of Paper and Paperboard. ASTM: West Conshohocken, PA, USA, 2002.
- Wu, H. Contribution to the chemistry of phosphomolybdic acids, phosphotungstic acids, and allied substances. J. Biol. Chem. 1920, 43, 189–220. [Google Scholar] [CrossRef]
- Rohrling, J.; Potthast, A.; Rosenau, T.; Lange, T.; Borgards, A.; Sixta, H.; Kosma, P. A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. 2. Validation and applications. Biomacromolecules 2002, 3, 969–975. [Google Scholar] [CrossRef]
- Bryantsev, V.S.; Diallo, M.S.; Goddard, W.A., 3rd. pKa calculations of aliphatic amines, diamines, and aminoamides via density functional theory with a Poisson-Boltzmann continuum solvent model. J. Phys. Chem. A 2007, 111, 4422–4430. [Google Scholar] [CrossRef] [PubMed]
- Jmol. Jmol: An Open Source Java Viewer for Chemical Structures in 3D. Undated. Available online: http://www.jmol.org/ (accessed on 13 November 2019).
- Zheng, Q.; Zhou, T.T.; Wang, Y.B.; Cao, X.H.; Wu, S.Q.; Zhao, M.L.; Wang, H.Y.; Xu, M.; Zheng, B.D.; Zheng, J.G.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep.-UK 2018, 8, 1321. [Google Scholar] [CrossRef]
- Rodriguez-Sanz, A.; Fucinos, C.; Torrado, A.M.; Rua, M.L. Extraction of the wheat straw hemicellulose fraction assisted by commercial endo-xylanases. Role of the accessory enzyme activities. Ind. Crop. Prod. 2022, 179, 114655. [Google Scholar] [CrossRef]
- Doan, H.D.; Lohi, A.; Dang, V.B.H.; Dang-Vu, T. Removal of Zn + 2 and Ni + 2 by adsorption in a fixed bed of wheat straw. Process Saf. Environ. 2008, 86, 259–267. [Google Scholar] [CrossRef]
- Pan, M.Z.; Zhao, G.M.; Ding, C.X.; Wu, B.; Lian, Z.N.; Lian, H.L. Physicochemical transformation of rice straw after pretreatment with a deep eutectic solvent of choline chloride/urea. Carbohydr. Polym. 2017, 176, 307–314. [Google Scholar] [CrossRef]
- ResinTech Product Specification Sheet, SIR-100-HP, Revision 1.1. Available online: https://www.resintech.com/wp-content/uploads/2023/01/SIR100HP_PDS.pdf (accessed on 14 September 2023).
- Chang, V.S.; Holtzapple, M.T. Fundamental factors affecting biomass enzymatic reactivity. Appl. Biochem. Biotech 2000, 84–86, 5–37. [Google Scholar] [CrossRef]
- Kong, F.R.; Engler, C.R.; Soltes, E.J. Effects of Cell-Wall Acetate, Xylan Backbone, and Lignin on Enzymatic-Hydrolysis of Aspen Wood. Appl. Biochem. Biotech. 1992, 34–35, 23–35. [Google Scholar] [CrossRef]
- Kumari, M.; Douglin, J.C.; Dekel, D.R. Crosslinked quaternary phosphonium-functionalized poly(ether ether ketone) polymer-based anion-exchange membranes. J. Membr. Sci. 2021, 626, 119167. [Google Scholar] [CrossRef]
- EPA Economics of Biofuels. Available online: https://www.epa.gov/environmental-economics/economics-biofuels#:~:text=Commercial%20cellulosic%20biofuel%20production%20began,are%20not%20yet%20produced%20commercially (accessed on 11 June 2023).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, S.E.; Ding, Y.; Sabatini, D.A.; Butler, E.C. Nitrate Uptake by Cellulose-Based Anion Exchange Polymers Derived from Wheat Straw. Water 2023, 15, 3594. https://0-doi-org.brum.beds.ac.uk/10.3390/w15203594
Jones SE, Ding Y, Sabatini DA, Butler EC. Nitrate Uptake by Cellulose-Based Anion Exchange Polymers Derived from Wheat Straw. Water. 2023; 15(20):3594. https://0-doi-org.brum.beds.ac.uk/10.3390/w15203594
Chicago/Turabian StyleJones, Sarah E., Yifan Ding, David A. Sabatini, and Elizabeth C. Butler. 2023. "Nitrate Uptake by Cellulose-Based Anion Exchange Polymers Derived from Wheat Straw" Water 15, no. 20: 3594. https://0-doi-org.brum.beds.ac.uk/10.3390/w15203594