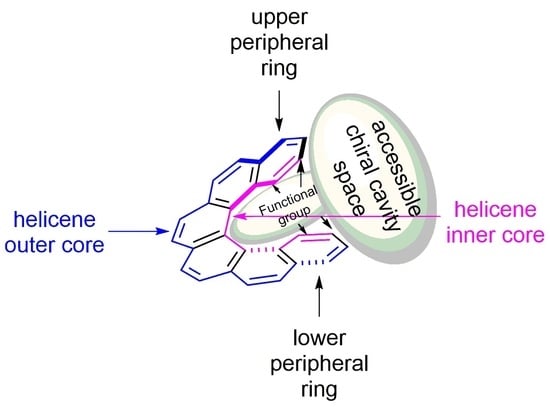
Helicene-Based Chiral Auxiliaries and Chirogenesis
Abstract
:1. Introduction
2. Overview of Latest Reviews
3. Classification and Nomenclature
3.1. Structural Diversity
3.1.1. Carbohelicenes
3.1.2. Heterohelicenes
3.1.3. Charged Helicenes
3.1.4. Special Structural Type of Helicene-Like Molecules
3.2. Spatial Diversity
3.2.1. Chiral (Configurationally Stable) Helicenes
3.2.2. Achiral Helicenes
3.2.3. Stereodynamic Helicenes
3.2.4. Meso Helicenes
4. General Description and Main Synthetic Approaches toward Helicene-Based Chirogenic Systems
5. Helicenes as Chiral Auxiliary/Reagent or Additive
6. Helicenes as Chiral Catalysts
7. Helicene in Supramolecular Chirogenesis
7.1. Helicene-Based Chiral Recognition of Small Molecules
7.2. Polymeric Helicene-Based Chiral Recognition
7.3. Helicene-Based Chiral Recognition via Nanoparticles
7.4. Helicene-Based Chiral Recognition Involving Biologically Relevant Molecules
7.5. Helicene-Based Chiral Recognition via Self-Assembly
8. Enantiopure Helicenes: Enantioselective Synthesis, Chiral Separation, and Racemization
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hembury, G.A.; Borovkov, V.V.; Inoue, Y. Chirality sensing supramolecular systems. Chem. Rev. 2008, 108, 1–73. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, C.F. Helicenes: Synthesis and applications. Chem. Rev. 2012, 112, 1463–1535. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M. One hundred years of helicene chemistry. Part 1: Non-stereoselective syntheses of carbohelicenes. Chem. Soc. Rev. 2013, 42, 968–1006. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M.; Félix, G.; Peresuttiab, R. One hundred years of helicene chemistry. Part 2: Stereoselective syntheses and chiral separations of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1007–1050. [Google Scholar] [CrossRef] [PubMed]
- Gingras, M. One hundred years of helicene chemistry. Part 3: Applications and properties of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1051–1095. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N. Photochemical reactions applied to the synthesis of helicenes and helicene-like compounds. J. Photochem. Photobiol. C Photochem. Rev. 2014, 19, 1–19. [Google Scholar] [CrossRef]
- Saleh, N.; Shena, C.; Crassous, J. Helicene-based transition metal complexes: Synthesis, properties and applications. Chem. Sci. 2014, 5, 3680–3694. [Google Scholar] [CrossRef]
- Aillard, P.; Voituriez, A.; Marinetti, A. Helicene-like chiral auxiliaries in asymmetric catalysis. Dalton Trans. 2014, 43, 15263–15278. [Google Scholar] [CrossRef] [PubMed]
- Virieux, D.; Sevrain, N.; Ayad, T.; Pirat, J.-L. Helical phosphorus derivatives. In Advances in Heterocyclic Chemistry; Eric, F.V.S., Christopher, A.R., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 116, pp. 37–83. [Google Scholar]
- Rajca, A.; Miyasaka, M. Functional Organic Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; Chapter 15; pp. 547–558. [Google Scholar]
- Chen, C.-F.; Shen, Y. Helicene Chemistry—From Synthesis to Applications; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-662-53168-6. [Google Scholar]
- Weitzenbock, R.; Lieb, H. New synthesis of chrysene. Monatshefte Chem. 1913, 33, 549–565. [Google Scholar] [CrossRef]
- Weitzenbock, R.; Klingler, A. Synthesis of the isomeric hydrocarbons 1,2,5,6-dibenzanthracene and 3,4,5,6-dibenzophenanthrene. J. Chem. Soc. 1918, 114, 494. [Google Scholar]
- Cook, J.W. Polycyclic aromatic hydrocarbons. Part XII. The orientation of derivatives of 1:2-benzanthracene, with notes on the preparation of some new homologues, and on the isolation of 3:4:5:6-dibenzphenanthrene. J. Chem. Soc. 1933, 0, 1592–1597. [Google Scholar] [CrossRef]
- Newman, M.S.; Lutz, W.B.; Lednicer, D. A new reagent for resolution by complex formation; the resolution of phenanthro-[3,4-c]phenanthrene. J. Am. Chem. Soc. 1955, 77, 3420–3421. [Google Scholar] [CrossRef]
- Newman, M.S.; Lednicer, D. The synthesis and resolution of hexahelicene. J. Am. Chem. Soc. 1956, 78, 4765–4770. [Google Scholar] [CrossRef]
- Morrison, D.J.; Trefz, T.K.; Piers, W.E.; McDonald, R.; Parvez, M. 7:8,9:10-Dibenzo-1,2,3,4-tetrafluoro-triphenylene: Synthesis, structure, and photophysical properties of a novel [5]helicene. J. Org. Chem. 2005, 70, 5309–5312. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Scott, L.T. Thermal cyclodehydrogenations to form 6-membered rings: Cyclizations of [5]helicenes. Org. Lett. 2007, 9, 3937–3940. [Google Scholar] [CrossRef] [PubMed]
- Minuti, L.; Taticchi, A.; Marrocchi, A.; Gacs-Baitz, E. Diels-Alder reaction of 3,3′,4,4′-tetrahydro-1,1′-binaphthalene. One-pot synthesis of a pentahelicenebenzoquinone. Tetrahedron 1997, 53, 6873–6878. [Google Scholar] [CrossRef]
- Ogawa, Y.; Ueno, T.; Tarikomi, M.; Seki, K.; Haga, K.; Uyehara, T. Synthesis of 2-acetoxy[5]helicene by sequential double aromatic oxy-Cope rearrangement. Tetrahedron Lett. 2002, 43, 7827–7829. [Google Scholar] [CrossRef]
- Pieters, G.; Gaucher, A.; Prim, D.; Marrot, J. First expeditious synthesis of 6,11-diamino-[6]carbohelicenes. Chem. Commun. 2009, 32, 4827–4828. [Google Scholar] [CrossRef] [PubMed]
- El Abed, R.; Aloui, F.; Genet, J.-P.; Ben Hassine, B.; Marinetti, A. Synthesis and resolution of 2-(diphenylphosphino)heptahelicene. J. Organomet. Chem. 2007, 692, 1156–1160. [Google Scholar] [CrossRef]
- Abbate, S.; Bazzini, C.; Caronna, T.; Fontana, F.; Gambarotti, C.; Gangemi, F.; Longhi, G.; Mele, A.; Sora, I.N.; Panzeri, W. Monoaza[5]helicenes. Part 2: Synthesis, characterisation and theoretical calculations. Tetrahedron 2006, 62, 139–148. [Google Scholar] [CrossRef]
- Meisenheimer, J.; Witte, K. Reduction of 2-nitronaphthalene. Chem. Ber. 1903, 36, 4153–4164. [Google Scholar] [CrossRef]
- Sundar, M.S.; Sahoo, S.; Bedekar, A.V. Synthesis and study of the structural properties of oxa[5]helicene derivatives. Tetrahedron Asymmetry 2016, 27, 777–781. [Google Scholar] [CrossRef]
- Dreher, S.D.; Weix, D.J.; Katz, T.J. Easy synthesis of functionalized hetero[7]helicenes. J. Org. Chem. 1999, 64, 3671–3678. [Google Scholar] [CrossRef] [PubMed]
- Eskildsen, J.; Krebs, F.C.; Faldt, A.; Sommer-Larsen, P.; Bechgaard, K. Preparation and structural properties of 7,8-dioxa[6]helicenes and 7a,14c-dihydro-7,8-dioxa[6]helicenes. J. Org. Chem. 2001, 66, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Oyama, H.; Nishimura, Y.; Nakasako, S.; Nozaki, K. k5-Phospha[7]helicenes: Synthesis, properties, and columnar aggregation with one-way chirality. Angew. Chem. Int. Ed. 2012, 51, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Oyama, H.; Nakano, K.; Harada, T.; Kuroda, R.; Naito, M.; Nobusawa, K.; Nozaki, K. Facile synthetic route to highly luminescent sila[7]helicene. Org. Lett. 2013, 15, 2104–2107. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Hashimoto, S.; Oba, T.; Nakamura, M. Azaboradibenzo[6]helicene: Carrier inversion induced by helical homochirality. J. Am. Chem. Soc. 2012, 134, 19600–19603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Edward, L.; Clennan, E.L.; Arulsamy, N. Photophysical and electrochemical characterization of a helical Viologen, N,N′-dimethyl-5,10-diaza[5]helicene. Org. Lett. 2014, 16, 4610–4613. [Google Scholar] [CrossRef] [PubMed]
- Guin, J.; Besnard, C.; Lacour, J. Synthesis, resolution, and stabilities of a cationic chromenoxanthene [4]helicene. Org. Lett. 2010, 12, 1748–1751. [Google Scholar]
- Rozen, S.; Dayan, S. At Last, 1,10-Phenanthroline-N,N′-dioxide, A new type of helicene, has been synthesized using HOF small middle dotCH(3)CN. Angew. Chem. Int. Ed. 1999, 38, 3471–3473. [Google Scholar] [CrossRef]
- Talele, H.R.; Sahoo, S.; Bedekar, A.V. Synthesis of chiral helical 1,3-oxazines. Org. Lett. 2012, 14, 3166–3169. [Google Scholar] [CrossRef] [PubMed]
- Jierry, L.; Harthong, S.; Aronica, C.; Mulatier, J.-C.; Guy, L.; Guy, S. Efficient dibenzo[c]acridine helicene-like synthesis and resolution: Scaleup, structural control, and high chiroptical properties. Org. Lett. 2012, 14, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Wynberg, H. Some observations on the chemical, photochemical, and spectral properties of thiophenes. Acc. Chem. Res. 1971, 4, 65–73. [Google Scholar] [CrossRef]
- Janke, R.H.; Haufe, G.; Würthwein, E.-U.; Borken, J.H. Racemization barriers of helicenes: A computational study. J. Am. Chem. Soc. 1996, 118, 6031–6035. [Google Scholar] [CrossRef]
- Groen, M.B.; Wynberg, H. Optical properties of some heterohelicenes. Absolute configuration. J. Am. Chem. Soc. 1971, 93, 2968–2974. [Google Scholar] [CrossRef]
- Fujikawa, T.; Segawa, Y.; Itami, K. Synthesis, structures, and properties of π-extended double helicene: A combination of planar and nonplanar π-systems. J. Am. Chem. Soc. 2015, 137, 7763–7768. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, P.; Xu, L.; Yang, J.; Shi, J.; Wang, Z.; Cheng, Y.; Wang, H. Synthesis for the mesomer and racemate of thiophene-based double helicene under irradiation. J. Org. Chem. 2013, 78, 6316–6321. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.S.; Wise, R.M. The synthesis and resolution of 1,12-dimethylbenzo[c]phenanthrene-5-acetic acid. J. Am. Chem. Soc. 1956, 78, 450–454. [Google Scholar] [CrossRef]
- Martin, R.H. The Helicenes. Angew. Chem. Int. Ed. 1974, 13, 649–660. [Google Scholar] [CrossRef]
- McCarthy, M.; Guiry, P.J. Axially chiral bidentate ligands in asymmetric catalysis. Tetrahedron 2001, 57, 3809–3844. [Google Scholar] [CrossRef]
- Chen, Y.; Yekta, S.; YudiA, K. Modified BINOL ligands in asymmetric catalysis. Chem. Rev. 2003, 103, 3155–3212. [Google Scholar] [CrossRef] [PubMed]
- Berthod, M.; Mignani, G.; Woodward, G.; Marc Lemaire, M. Modified BINAP: The how and the why. Chem. Rev. 2005, 105, 1801–1836. [Google Scholar] [CrossRef] [PubMed]
- Hassine, B.B.; Gorsane, M.; Pecher, J.; Martin, R.H. Diastereoselective sodium borohydride reductions of (dl) α-keto esters. Bull. Soc. Chim. Belg. 1985, 94, 597–603. [Google Scholar] [CrossRef]
- Hassine, B.B.; Gorsane, M.; Geerts-Evrard, F.; Pecher, J.; Martin, R.H.; Castelet, D. Payne syntheses and enantioselective syntheses of epoxides from (E)-stilbene and α-methylstyrene. Bull. Soc. Chim. Belg. 1986, 95, 557–566. [Google Scholar] [CrossRef]
- Hassine, B.B.; Gorsane, M.; Pecher, J.; Martin, R.H. Potential asymmetric syntheses via the ene reaction. Bull. Soc. Chim. Belg. 1987, 96, 801–808. [Google Scholar]
- Hassine, B.B.; Gorsane, M.; Pecher, J.; Martin, R.H. Asymmetric syntheses and potential asymmetric synthesis of α-amino alcohols: Hydroxyamination of olefins by the Sharpless method. Bull. Soc. Chim. Belg. 1985, 94, 759–769. [Google Scholar] [CrossRef]
- Hassine, B.B.; Gorsane, M.; Pecher, J.; Martin, R.H. Atrolactic synthesis in the evaluation of the efficiency of inducers of asymmetric synthesis. Bull. Soc. Chim. Belg. 1986, 95, 547–556. [Google Scholar]
- Yamamoto, K.; Shimizu, T.; Igawa, K.; Tomooka, K.; Hirai, G.; Suemune, H.; Usui, K. Rational design and synthesis of [5]helicene-derived phosphine ligands and their application in Pd-catalyzed asymmetric reactions. Sci. Rep. 2016, 6, 36211. [Google Scholar] [CrossRef] [PubMed]
- Tsujihara, T.; Inada-Nozaki, N.; Takehara, T.; Zhou, D.-Y.; Suzuki, T.; Kawano, T. Nickel-catalyzed construction of chiral 1-[6]helicenols and application in the synthesis of [6]helicene-based phosphinite ligands. Eur. J. Org. Chem. 2016, 2016, 4948–4952. [Google Scholar] [CrossRef]
- Reetz, M.T.; Sostmann, S. Kinetic resolution in Pd-catalyzed allylic substitution using the helical PHelix ligand. J. Organomet. Chem. 2000, 603, 105–109. [Google Scholar] [CrossRef]
- Reetz, M.T.; Sostmann, S. First enantioselective catalysis using a helical diphosphane. Tetrahedron Lett. 1997, 38, 3211–3214. [Google Scholar] [CrossRef]
- Nakano, D.; Yamaguchi, M. Enantioselective hydrogenation of itaconate using rhodium bihelicenol phosphite complex. Matched/mismatched phenomena between helical and axial chirality. Tetrahedron Lett. 2003, 44, 4969–4971. [Google Scholar] [CrossRef]
- Yavari, K.; Aillard, P.; Zhang, Y.; Nuter, F.; Retailleau, P.; Voituriez, A.; Marinetti, A. Helicenes with embedded phosphole units in enantioselective gold catalysis. Angew. Chem. Int. Ed. 2014, 53, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Aillard, P.; Voituriez, A.; Dova, D.; Cauteruccio, S.; Licandro, E.; Marinetti, A. Phosphathiahelicenes: Synthesis and uses in enantioselective gold catalysis. Chem. Eur. J. 2014, 20, 12373–12376. [Google Scholar] [CrossRef] [PubMed]
- Cauteruccio, S.; Dova, D.; Benaglia, M.; Genoni, A.; Orlandi, M.; Licandro, E. Synthesis, characterisation, and organocatalytic activity of chiral tetrathiahelicene diphosphine oxides. Eur. J. Org. Chem. 2014, 2014, 2694–2702. [Google Scholar] [CrossRef]
- Šamal, M.; Misek, J.; Stara, I.G.; Stary, I. Organocatalysis with azahelicenes: The first use of helically chiral pyridine-based catalysts in the asymmetric acyl transfer reaction. Collect. Czechoslov. Chem. Commun. 2009, 74, 1151–1159. [Google Scholar] [CrossRef]
- Misek, J.; Teply, F.; Stara, I.G.; Tichy, M.; Saman, D.; Cisarova, I.; Vojtisek, P.; Stary, I. A straightforward route to helically chiral N-heteroaromatic compounds: Practical synthesis of racemic 1,14-diaza[5]helicene and optically pure 1- and 2-aza[6]helicenes. Angew. Chem. Int. Ed. 2008, 47, 3188–3191. [Google Scholar] [CrossRef] [PubMed]
- Crittall, M.R.; Rzepa, H.S.; Carbery, D.R. Design, synthesis, and evaluation of a helicenoidal DMAP Lewis base catalyst. Org. Lett. 2011, 13, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Ruble, J.C.; Latham, H.A.; Fu, G.C. Effective kinetic resolution of secondary alcohols with a planar−chiral analogue of 4-(Dimethylamino)pyridine. Use of the Fe(C5Ph5) group in asymmetric catalysis. J. Am. Chem. Soc. 1997, 119, 1492–1493. [Google Scholar] [CrossRef]
- Ruble, J.C.; Tweddell, J.; Fu, G.C. Kinetic resolution of arylalkylcarbinols catalyzed by a planar-chiral derivative of DMAP: A new benchmark for nonenzymatic acylation. J. Org. Chem. 1998, 63, 2794–2795. [Google Scholar] [CrossRef]
- Spivey, A.C.; Fekner, T.; Spey, S.E. Axially chiral analogues of 4-(Dimethylamino)pyridine: Novel catalysts for nonenzymatic enantioselective acylations. J. Org. Chem. 2000, 65, 3154–3159. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Takenaka, N. Helical chiral pyridine N-oxides: A new family of asymmetric catalysts. Chem. Eur. J. 2009, 15, 7268–7276. [Google Scholar] [CrossRef] [PubMed]
- Narcis, M.J.; Takenaka, N. Helical-chiral small molecules in asymmetric catalysis. Eur. J. Org. Chem. 2014, 2014, 21–34. [Google Scholar] [CrossRef]
- Takenaka, N.; Sarangthem, R.S.; Captain, B. Helical chiral pyridine N-oxides: A new family of asymmetric catalysts. Angew. Chem. Int. Ed. 2008, 47, 9708–9710. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Captain, B.; Takenaka, N. Helical chiral 2,20-bipyridine N-monoxides as catalysts in the enantioselective propargylation of aldehydes with allenyltrichlorosilane. Org. Lett. 2011, 13, 1654–1657. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhu, R.; An, Y.; Wheeler, S.E. Origin of enantioselectivity in the propargylation of aromatic aldehydes catalyzed by helical N-oxides. J. Am. Chem. Soc. 2012, 134, 3095–3102. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, N.; Chen, J.; Captain, B.; Sarangthem, R.S.; Chandrakumar, A. Helical chiral 2-aminopyridinium ions: A new class of hydrogen bond donor catalysts. J. Am. Chem. Soc. 2010, 132, 4536–4537. [Google Scholar] [CrossRef] [PubMed]
- Narcis, M.J.; Sprague, D.J.; Captain, B.; Takenaka, N. Enantio- and periselective nitroalkene Diels–Alder reaction. Org. Biomol. Chem. 2012, 10, 9134–9136. [Google Scholar] [CrossRef] [PubMed]
- Sato, I.; Yamashima, R.; Kadowaki, K.; Yamamoto, J.; Shibata, T.; Soai, K. Asymmetric induction by helical hydrocarbons: [6]- and [5]helicenes. Angew. Chem. Int. Ed. 2001, 40, 1096–1098. [Google Scholar] [CrossRef]
- Kawasaki, T.; Suzuki, K.; Licandro, E.; Bossi, A.; Maiorana, S.; Soai, K. Enantioselective synthesis induced by tetrathia-[7]-helicenes in conjunction with asymmetric autocatalysis. Tetrahedron Asymmetry 2006, 17, 2050–2053. [Google Scholar] [CrossRef]
- Dreher, S.D.; Katz, T.J.; Lam, K.-C.; Rheingold, A.L. Application of the Russig-Laatsch reaction to synthesize a bis[5]helicene chiral pocket for asymmetric catalysis. J. Org. Chem. 2000, 65, 815–822. [Google Scholar] [CrossRef]
- Amemiya, R.; Yamaguchi, M. Chiral recognition in noncovalent bonding interactions between helicenes: Right-handed helix favors right-handed helix over left-handed helix. Org. Biomol. Chem. 2008, 6, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Yamaguchi, M.; Kabuto, C. Macrocyclic amides consisting of helical chiral 1,12-dimethylbenzo[c]phenanthrene-5,8-dicarboxylate. J. Org. Chem. 1998, 63, 9500–9509. [Google Scholar] [CrossRef]
- Reetz, M.T.; Sostmann, S. 2,15-Dihydroxy-hexahelicene (HELIXOL): Synthesis and use as an enantioselective fluorescent sensor. Tetrahedron 2001, 57, 2515–2520. [Google Scholar] [CrossRef]
- Nakazaki, M.; Yamamoto, K.; Ikeda, T.; Kitsuki, T.; Okamoto, Y. Synthesis and chiral recognition of novel crown ethers incorporating helicene chiral centres. J. Chem. Soc. Chem. Commun. 1983, 0, 787–788. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ikeda, T.; Kitsuki, T.; Okamoto, Y.; Chikamatsu, H.; Nakazaki, M. Synthesis and chiral recognition of optically active crown ethers incorporating a helicene moiety as the chiral centre. J. Chem. Soc. Perkin Trans. 1 1990, 0, 271–276. [Google Scholar] [CrossRef]
- Pandey, A.D.; Mohammed, H.; Pissurlenkar, R.R.S.; Karnik, A.V. Size-induced chiral discrimination switching by (S)-(-)-2(α-hydroxyethyl)benzimidazole-derived azacrowns. ChemPlusChem 2015, 80, 475–479. [Google Scholar] [CrossRef]
- Weix, D.J.; Dreher, S.D.; Katz, T.J. [5]HELOL phosphite: A helically grooved sensor of remote chirality. J. Am. Chem. Soc. 2000, 122, 10027–10032. [Google Scholar] [CrossRef]
- Wang, D.Z.; Katz, T.J. A [5]HELOL analogue that senses remote chirality in alcohols, phenols, amines, and carboxylic acids. J. Org. Chem. 2005, 70, 8497–8502. [Google Scholar] [CrossRef] [PubMed]
- Schweinfurth, D.; Zalibera, M.; Kathan, M.; Shen, C.; Mazzolini, M.; Trapp, N.; Crassous, J.; Gescheidt, G.; Diederich, F. Helicene quinones: Redox-triggered chiroptical switching and chiral recognition of the semiquinone radical anion lithium salt by electron nuclear double resonance spectroscopy. J. Am. Chem. Soc. 2014, 136, 13045–13052. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Khose, V.N.; Mori, T.; Borovkov, V.; Karnik, A.V. Sui generis helicene-based supramolecular chirogenic system: Enantioselective sensing, solvent control, and application in chiral group transfer reaction. ACS Omega 2017, 2, 592–598. [Google Scholar] [CrossRef]
- Hasan, M.; Pandey, A.D.; Khose, V.N.; Mirgane, N.A.; Karnik, A.V. Sterically congested chiral 7,8-dioxa[6]helicene and Its dihydro analogues: Synthesis, regioselective functionalization, and unexpected domino Prins reaction. Eur. J. Org. Chem. 2015, 2015, 3702–3712. [Google Scholar] [CrossRef]
- Hasan, M.; Khose, V.N.; Pandey, A.D.; Borovkov, V.; Karnik, A.V. Tailor-made supramolecular chirogenic system based on Cs-symmetric rigid organophosphoric acid host and amino alcohols: Mechanistic studies, bulkiness effect, and chirality sensing. Org. Lett. 2016, 18, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Anger, E.; Iida, H.; Yamaguchi, T.; Hayashi, K.; Kumano, D.; Crassous, J.; Vanthuyne, N.; Roussel, C.; Yashima, E. Synthesis and chiral recognition ability of helical polyacetylenes bearing helicene pendants. Polym. Chem. 2014, 5, 4909–4914. [Google Scholar] [CrossRef]
- An, Z.; Yamaguchi, M. Chiral recognition in aggregation of gold nanoparticles grafted with helicenes. Chem. Commun. 2012, 48, 7383–7385. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, W.; Miyagawa, M.; An, Z.; Yamaguchi, M. Optical resolution of aromatic alcohols using silica nanoparticles grafted with helicene. Org. Lett. 2012, 14, 3123–3125. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, M.; Ichinose, W.; Yamaguchi, M. Equilibrium shift in solution: Molecular shape recognition and precipitation of a synthetic double helix using helicene-grafted silica nanoparticles. Chem. Eur. J. 2014, 20, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Ishii, R.; Nakagawa, H.; Kawazura, H. Inclusion of Trithia[5]heterohelicene by various serum albumins. An effective probe for chiral discrimination. Chem. Pharm. Bull. 1997, 45, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Kobori, Y.; Yamada, K. Spontaneous chirality conversion of [5]thiaheterohelicene in charge-transfer complexes in SDS micelles. Chirality 2001, 13, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Gomi, K.; Yamada, K. Chiral recognition of thiaheterohelicenes by alkyl b-D-pyranoside micelles. Influence of extension of helix. Chem. Pharm. Bull. 2001, 49, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Kobori, Y.; Yoshida, M.; Yamada, K. Chiral recognition by single bilayered phosphatidylcholine vesicles using [5]thiaheterohelicene as a probe. Chem. Commun. 2001, 0, 2692–2693. [Google Scholar] [CrossRef]
- Nakagawa, H.; Yoshida, M.; Kobori, Y.; Yamada, K. Study of chiral recognition of bilayered phosphatidylcholine vesicles using a helicene probe: Characteristic function of cholesterol. Chirality 2003, 15, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Yamada, K. Difference in chiral recognition of gel and liquid-crystalline phases of phosphatidylcholine vesicles as determined by circular dichroism spectroscopy. Chem. Pharm. Bull. 2005, 53, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Osuga, H.; Suzuki, H.; Shogase, Y.; Kitahara, Y. Synthesis, enzymic resolution and enantiomeric enhancement of bis(hydroxymethyl)[7]thiaheterohelicenes. J. Chem. Soc. Perkin Trans. 1 1998, 0, 935–940. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.X.; Sugiyama, H.; Umano, T.; Osuga, H.; Tanaka, K. (P)-Helicene displays chiral selection in binding to Z-DNA. J. Am. Chem. Soc. 2004, 126, 6566–6567. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Osuga, H.; Kitahara, Y. Elongation and contraction of molecular springs. Synthesis, structures, and properties of bridged [7]thiaheterohelicenes. J. Org. Chem. 2002, 67, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K.; Sannohe, Y.; Kaieda, S.; Tanaka, K.; Osuga, H.; Tahara, H.; Xu, Y.; Kawase, T.; Bando, T.; Sugiyama, H. A chiral wedge molecule inhibits telomerase activity. J. Am. Chem. Soc. 2010, 132, 3778–3782. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, G.; Kawakami, K.; Sasaki, S. Enantioselective binding of chiral 1,14-dimethyl[5]helicene–spermine ligands with B- and Z-DNA. Bioorg. Med. Chem. 2013, 21, 6063–6068. [Google Scholar] [CrossRef] [PubMed]
- Kel, O.; Frstenberg, A.; Mehanna, N.; Nicolas, C.; Laleu, B.; Hammarson, M.; Albinsson, B.; Lacour, J.; Vauthey, E. Chiral selectivity in the binding of [4]helicene derivatives to double-stranded DNA. Chem. Eur. J. 2013, 19, 7173–7180. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jiang, L.; Liang, W.; Gui, J.; Xu, D.; Wu, W.; Nakai, Y.; Nishijima, M.; Fukuhara, G.; Mori, T.; et al. Inherently chiral azonia[6]helicene-modified β-cyclodextrin: Synthesis, characterization, and chirality sensing of underivatized amino acids in water. J. Org. Chem. 2016, 81, 3430–3434. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Nakano, D.; Anzai, S.; Yamaguchi, M. Synthesis of symmetrical polynitrohelicenes and their chiral recognition in the charge transfer complexation. J. Org. Chem. 2001, 66, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Murguly, E.; McDonald, R.; Branda, N.R. Chiral discrimination in hydrogen-bonded [7]helicenes. Org. Lett. 2000, 2, 3169–3172. [Google Scholar] [CrossRef] [PubMed]
- Mikes, F.; Boshart, G.; Gil-Av, E. Resolution of optical isomers by high-performance liquid chromatography, using coated and bonded chiral charge-transfer complexing agents as stationary phases. J. Chromatogr. A 1976, 122, 205–221. [Google Scholar] [CrossRef]
- Thongpanchang, T.; Paruch, K.; Katz, T.J.; Rheingold, A.R.; Lam, K.-C.; Liable-Sands, L. Why (1S)-camphanates are excellent resolving agents for helicen-1-ols and why they can be used to analyze absolute configurations. J. Org. Chem. 2000, 65, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Bucinskas, A.; Waghray, D.; Bagdziunas, G.; Thomas, J.; Grazulevicius, J.V.; Dehaen, W. Synthesis, functionalization, and optical properties of chiral carbazole-based diaza[6]helicenes. J. Org. Chem. 2015, 80, 2521–2528. [Google Scholar] [CrossRef] [PubMed]
- Waghray, D.; Bagdziunas, G.; Jacobs, J.; Meervelt, L.V.; Grazulevicius, J.V.; Dehaen, W. Diastereoselective strategies towards thia[n]helicenes. Chem. Eur. J. 2015, 21, 18791–18798. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Hidehira, Y.; Takahashi, K.; Hiyama, T.; Nozaki, K. Stereospecific synthesis of hetero[7]helicenes by Pd-catalyzed double N-arylation and intramolecular O-arylation. Angew. Chem. Int. Ed. 2005, 44, 7136–7138. [Google Scholar] [CrossRef] [PubMed]
- Sako, M.; Takeuchi, Y.; Tsujihara, T.; Kodera, J.; Kawano, T.; Takizawa, S.; Sasai, T. Efficient enantioselective synthesis of oxahelicenes using redox/acid cooperative catalysts. J. Am. Chem. Soc. 2016, 138, 11481–11484. [Google Scholar] [CrossRef] [PubMed]
- Šámal, M.; Chercheja, S.; Rybáček, J.; Chocholoušová, J.V.; Vacek, J.; Bednárová, L.; Šaman, D.; Stará, I.G.; Starý, I. An ultimate stereocontrol in ssymmetric synthesis of optically pure fully aromatic helicenes. J. Am. Chem. Soc. 2015, 137, 8469–8474. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.; Cabellos, J.L.; Pan, S.; Murillo, F.; Zarate, X.; Fernandez-Herrera, M.A.; Merino, G. Revisiting the racemization mechanism of helicenes. Chem. Commun. 2018, 54, 188–191. [Google Scholar] [CrossRef] [PubMed]















| (P)-53 | [(η3-C3H5)-PdCl]2 (2.5 mol%), (P)-53 (10 mol%), BSA, LiOAc, CH2Cl2, rt, 24 h | 90 | 96 | [52] |
| (P)-54 | [(η3-C3H5)-PdCl]2 (2.5 mol%), (P)-54 (10 mol%), BSA, LiOAc, CH2Cl2, rt, 24 h | 97 | 99 | [52] |













































© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.; Borovkov, V. Helicene-Based Chiral Auxiliaries and Chirogenesis. Symmetry 2018, 10, 10. https://0-doi-org.brum.beds.ac.uk/10.3390/sym10010010
Hasan M, Borovkov V. Helicene-Based Chiral Auxiliaries and Chirogenesis. Symmetry. 2018; 10(1):10. https://0-doi-org.brum.beds.ac.uk/10.3390/sym10010010
Chicago/Turabian StyleHasan, Mohammed, and Victor Borovkov. 2018. "Helicene-Based Chiral Auxiliaries and Chirogenesis" Symmetry 10, no. 1: 10. https://0-doi-org.brum.beds.ac.uk/10.3390/sym10010010